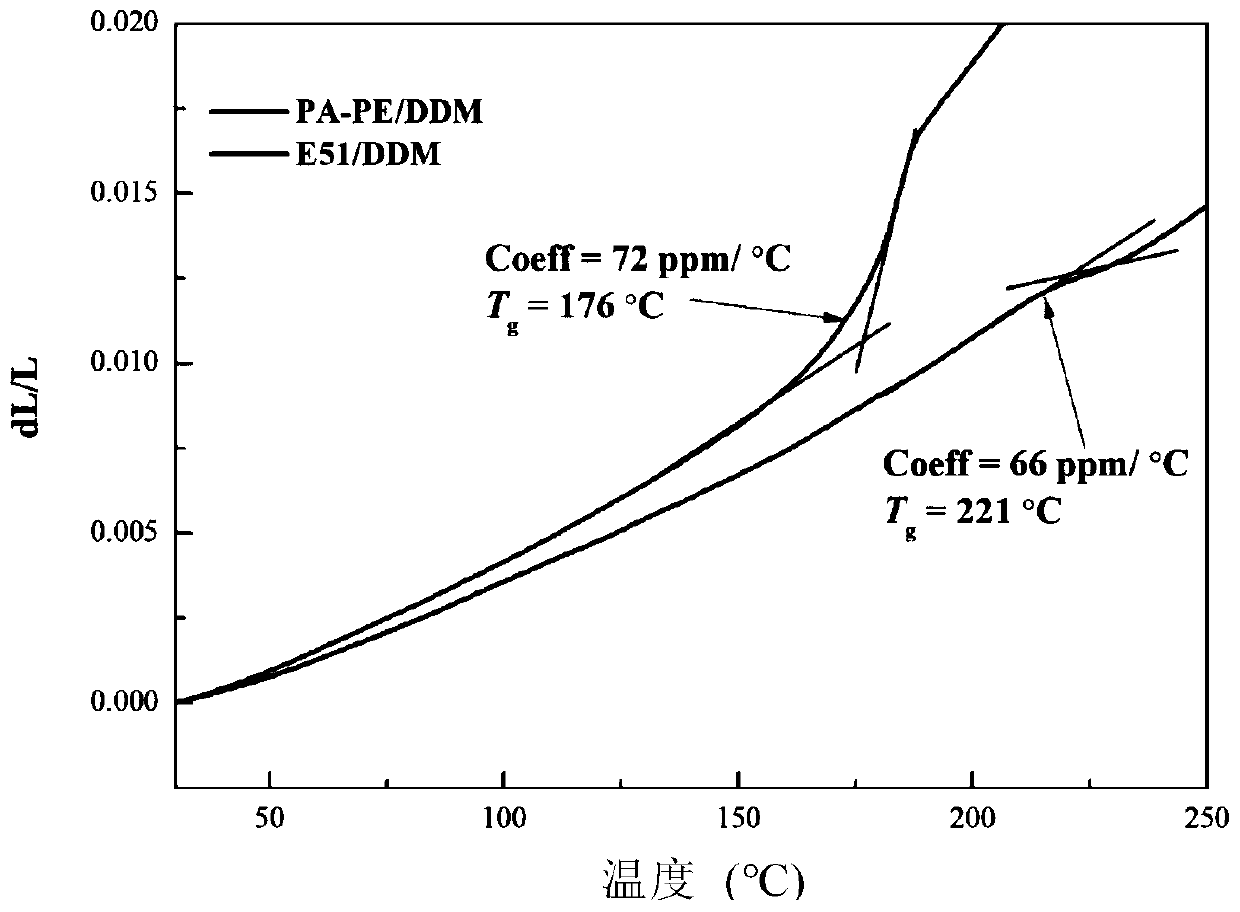
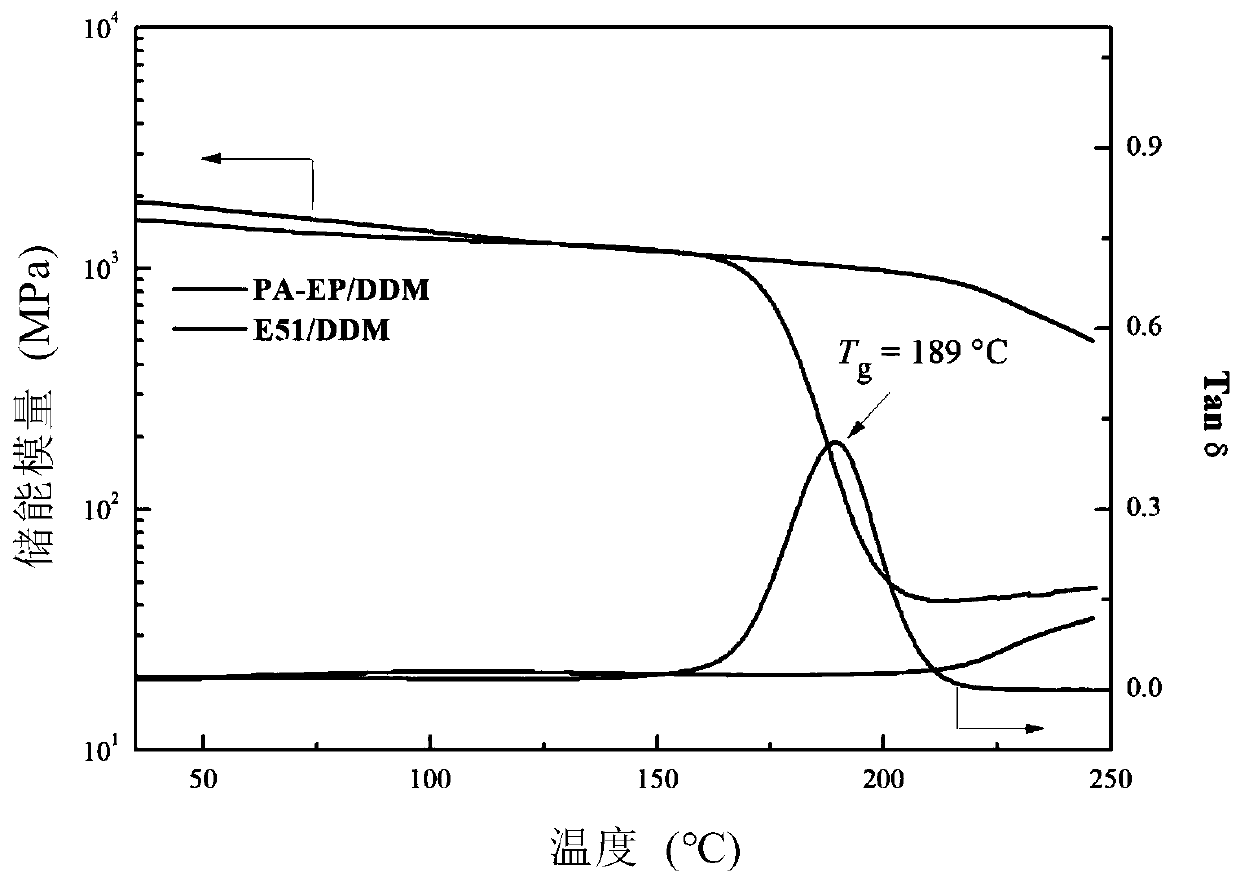
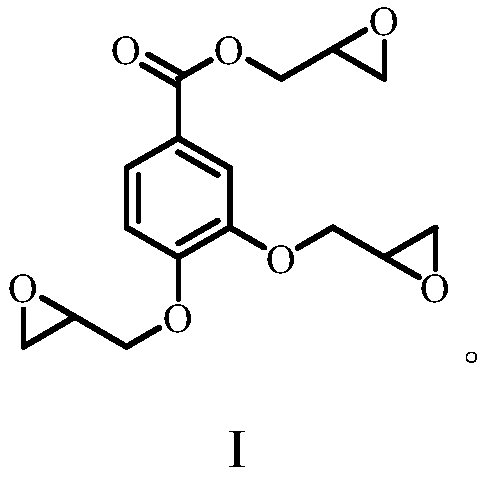
Synthesis and application of epoxy resin based on catechuic acid
A technology of catechin epoxy resin and epoxy resin, applied in the direction of organic chemistry, etc., can solve many problems such as use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The synthesis of embodiment 1 protocatechuic acid epoxy monomer
[0052] Under the protection of nitrogen gas, add magneton, 100mmol protocatechuic acid and 94mL epichlorohydrin to a 250ml dry three-necked bottle. Then 5 mmol of triethylbenzylammonium chloride was added. Raise the temperature to 100°C and react for 2 hours, cool to room temperature, add 120 mL of 20 wt% sodium hydroxide aqueous solution, react at room temperature for 2 hours, add water to wash, extract with ethyl acetate several times, dry with anhydrous sodium sulfate, concentrate, and column chromatography to obtain the target 6.4 g of a colorless oily epoxy compound monomer.
[0053] 1 H NMR (400MHz, DMSO-d 6 )δ7.62(dd, J=8.4,2.0Hz,1H),7.52(d,J=2.1Hz,1H),7.14(d,J=8.6Hz,1H),4.61(dd,J=12.4,2.7 Hz,1H),4.50–4.39(m,2H),4.06(dd,J=12.4,6.4Hz,1H),3.92(dddd,J=21.9,11.4,6.6,1.8Hz,2H),3.39(dqd, J=7.9,4.0,2.2Hz,3H),2.90–2.81(m,3H),2.74(ddt,J=7.3,5.0,2.7Hz,3H). 13 C NMR (101MHz, CDCl 3 )δ165.71, 152.74, 14...
Embodiment 2
[0054] The synthesis of embodiment 2 protocatechuic acid epoxy monomer
[0055] The experimental method was the same as that in Example 1, except that tetraethylammonium chloride was used instead of triethylbenzylammonium chloride, and 5.2 g of the target monomer was obtained by column chromatography.
Embodiment 3
[0056] The synthesis of embodiment 3 protocatechuic acid epoxy monomer
[0057] The experimental method was the same as that in Example 1, except that tetrabutylammonium chloride was used instead of triethylbenzylammonium chloride, and 5.1 g of the target monomer was obtained by column chromatography.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com