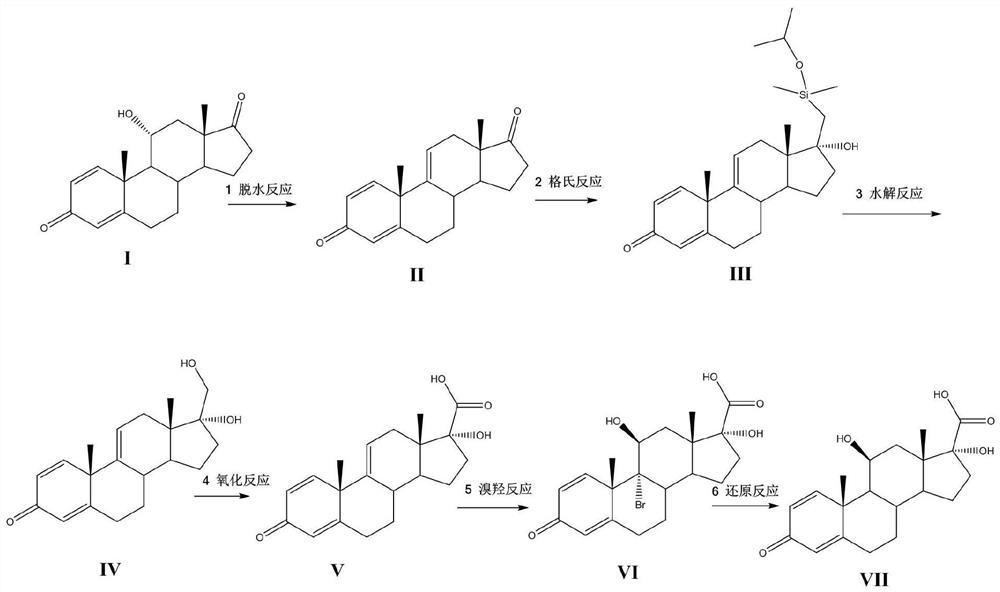
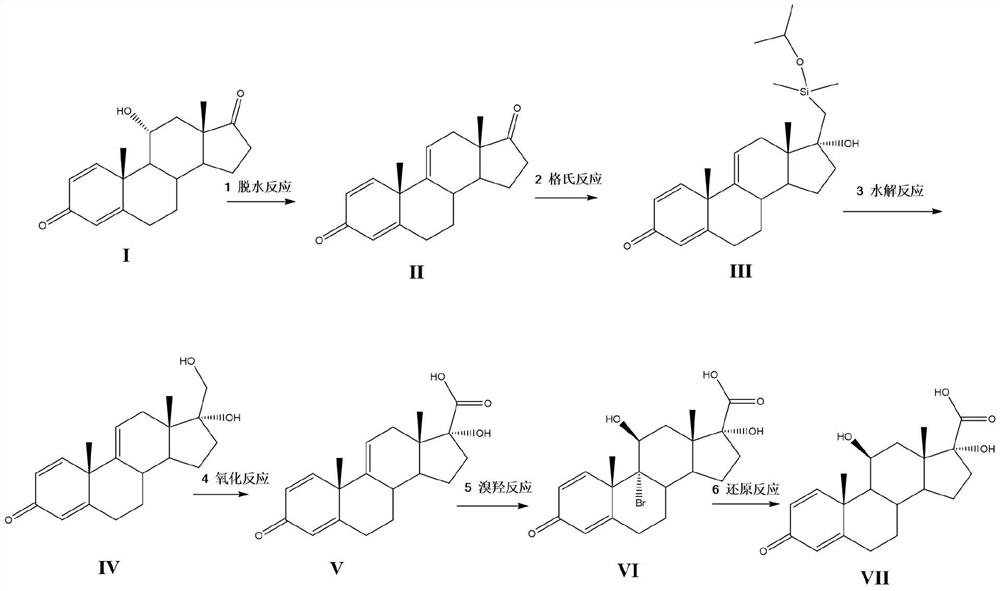
A kind of synthetic method of loteprednol intermediate
A technology of loteprednol etabon and intermediates, which is applied in the field of preparation of loteprednol etabon, can solve the problem of high cost of raw materials, and achieve the effects of good purity, high yield and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis of Compound II
Embodiment 1-1
[0038] Under the protection of nitrogen, 15g 11α hydroxy-ADD (Compound I), 100ml of dichloromethane, 2ml of acetic anhydride were added to the reaction bottle, the control temperature was dropped into 10ml of phosphoric acid at 0 ~ 5 ° C, after 5 hours of insulation stirring, diluted to 10% sodium bicarbonate aqueous solution neutralized, stirred for 30 minutes, filtered, dried to give 13.6g yellow solid, molar yield 96.5%, HPLC purity 93%.
Embodiment 1-2
[0040] Under the protection of nitrogen, 100ml of tetrahydrofuran, 5g of phosphorus pentoxide was added to the reaction bottle, 15g of 11α hydroxy-ADD (Compound I) was added at a controlled temperature of 0 to 5 °C, after 5 hours of insulation and agitation, diluted to 10% aqueous sodium bicarbonate solution for neutralization, stirred for 30 minutes, filtered, dried to give 13.1g of compound II, molar yield of 92.9%, HPLC purity of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com