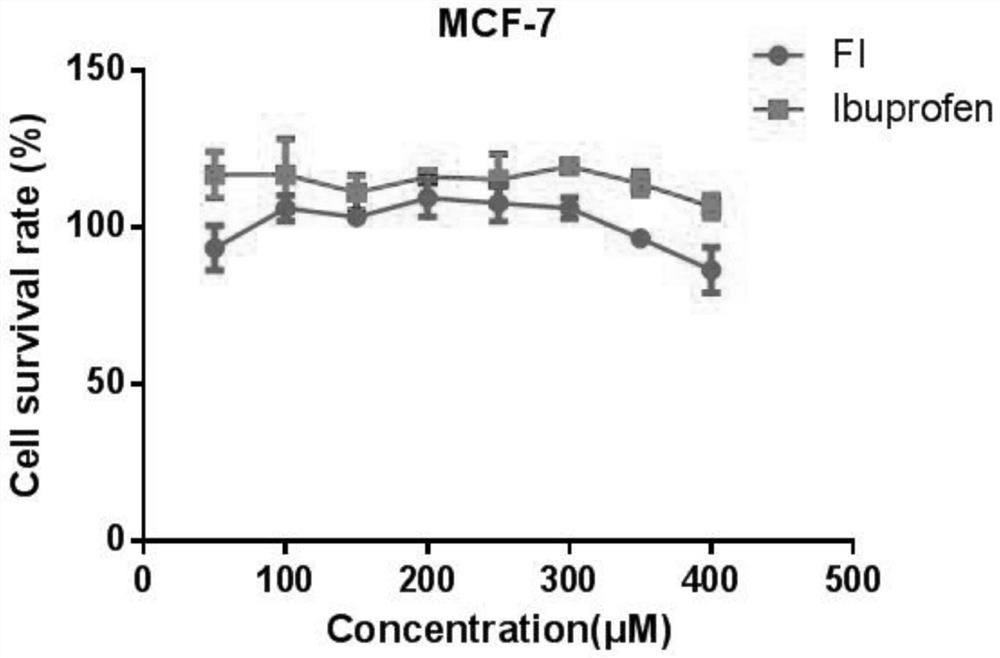
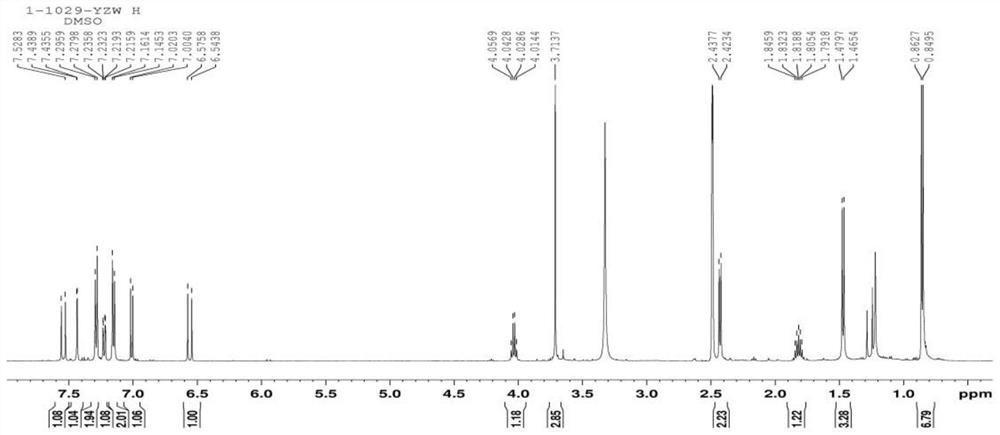
Synthesis method of ibuprofen ferulate and its application in preparation of immunosuppressive drugs
A ferulic acid ester and drug technology, applied in the field of organic synthesis, can solve problems such as the unexplained anti-tumor mechanism of ibuprofen, and achieve the effect of convenient industrial transformation and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthetic method of the ibuprofen ferulate of the present embodiment is as follows:
[0027] (1) Add 2.06 g (10 mmol) of ibuprofen, 20 mL of anhydrous dichloromethane and 1 drop of pyridine to the three-necked flask in sequence. Slowly add SOCl dropwise while stirring at room temperature 2 (0.5 mL) of CH 2 Cl 2 Solution 5mL. The temperature of the oil bath was kept at 60° C. and refluxed for 2 hours. Slowly cool the reaction solution afterwards, remove excess SOCl under reduced pressure 2 , spin-dried to obtain a light yellow solid, add 5 mL of anhydrous dichloromethane, and seal it for later use;
[0028] Methanol-derived ester method to check whether the acid chloride was prepared successfully. Take a little methanol into a clean 1.5ml EP tube, add a small amount of ibuprofen acid chloride dropwise and mix well, and confirm that the acid chloride is successfully prepared by TLC spotting;
[0029] (2) Take another three-necked bottle and add 0.194g (1mmol) o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com