Toxoplasma gondii IgG antibody detection chemiluminescence immune assay determination kit and preparation method thereof
A chemiluminescence immunoassay and chemiluminescence technology, applied in the field of immunoassay, can solve the problems of short validity period of reagents, high detection cost, cumbersome operation, etc., and achieve the effects of improved sensitivity and accuracy, wide linear range, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention also provides a preparation method of a chemiluminescence immunoassay assay kit for detecting IgG antibodies to toxoplasma gondii, comprising the following steps:
[0040] 1. Preparation of calibrator
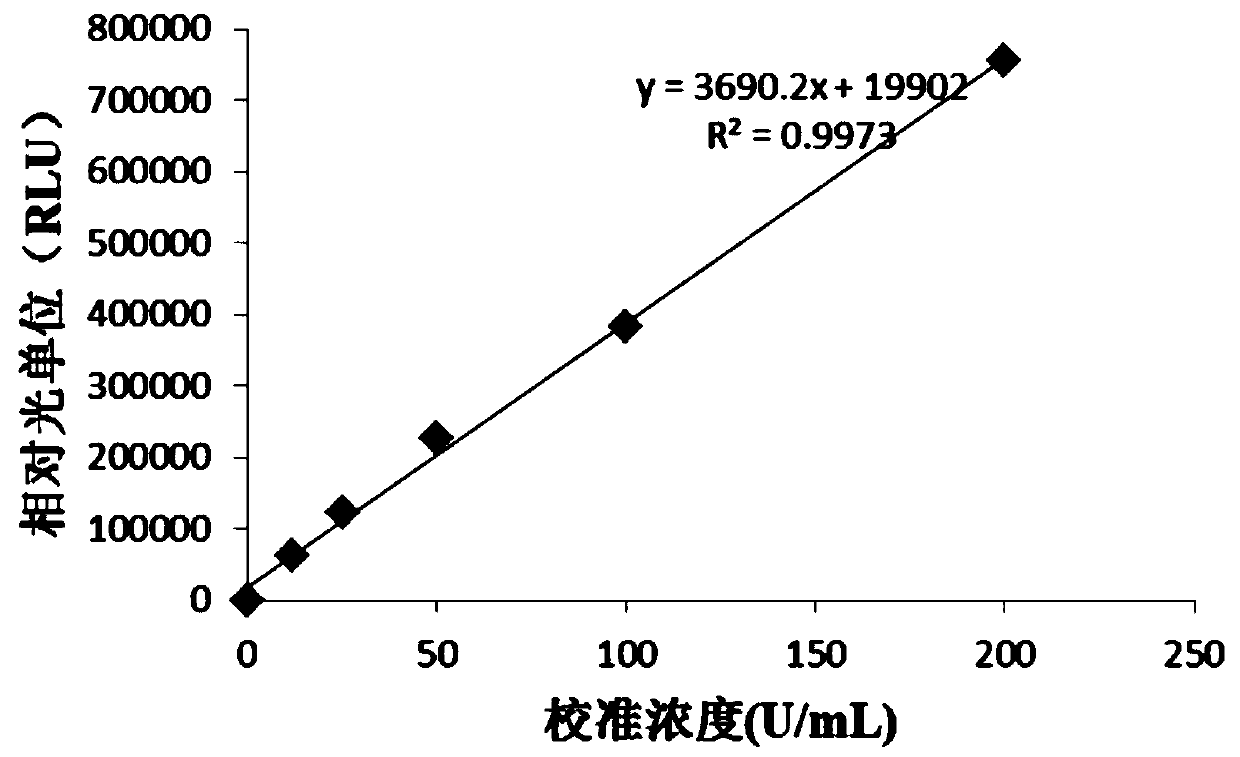
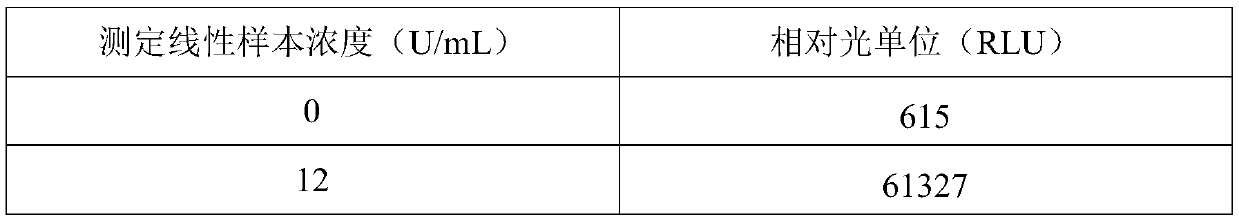
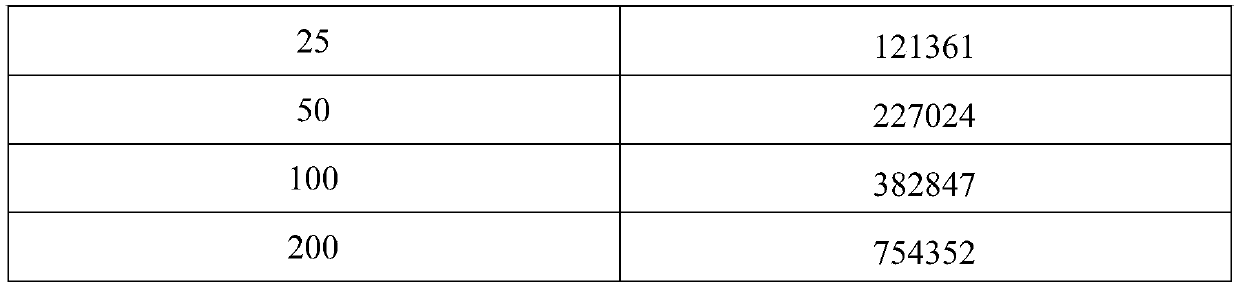
[0041] Dilute the TOXO IgG antibody into a calibrator with 100mM PBS buffer containing 20% calf serum, and aliquot into 0U / mL, 12U / mL, 25U / mL, 50U / mL, 100U / mL, 200U / mL;
[0042] 2. Preparation of antigen R1 solution coated with magnetic beads
[0043]Use a vortex mixer to thoroughly mix the magnetic beads, and add 0.5-1.5 mL of the suspended liquid (10 mg / mL magnetic beads) to the centrifuge tube. Place the centrifuge tube on the magnetic separation rack for 1-3min, carefully remove the supernatant, add 1mL of blocking agent, mix thoroughly with a vortex mixer, and then place the centrifuge tube on the magnetic separation rack for 1-3min (or longer), carefully remove the supernatant, repeat the blocking step for a total of 3 times, add 1mL of bl...
Embodiment 1
[0052] 1) Preparation of calibrator
[0053] Dilute the TOXO IgG antibody into a calibrator with 100mM PBS buffer containing 20% calf serum, and aliquot into 0U / mL, 12U / mL, 25U / mL, 50U / mL, 100U / mL, 200U / mL.
[0054] 2) Preparation of magnetic bead-coated antigen R1 solution
[0055] Use a vortex mixer to thoroughly mix the magnetic beads, and add 0.5 mL of the suspended liquid (10 mg / mL magnetic beads) to the centrifuge tube. Place the centrifuge tube on the magnetic separation rack for 1 min, and carefully remove the supernatant. Add 1mL of blocking agent, use a vortex mixer to mix thoroughly, then place the centrifuge tube on the magnetic separation rack for 1min (or longer), and carefully remove the supernatant. Repeat the blocking step a total of 3 times. Add 1 mL of blocking agent again, and mix well using a vortex mixer. Add 50 μg of coating antibody and vortex to mix well. Use a 360° rotary mixer to incubate at room temperature for 2 h. Place the centrifuge tube...
Embodiment 2
[0061] 1) Preparation of calibrator
[0062] Dilute the TOXO IgG antibody into a calibrator with 100mM PBS buffer containing 20% calf serum, and aliquot into 0U / mL, 12U / mL, 25U / mL, 50U / mL, 100U / mL, 200U / mL.
[0063] 2) Preparation of antigen R1 solution coated with magnetic beads
[0064] Use a vortex mixer to thoroughly mix the magnetic beads, and add 1 mL of the suspended liquid (10 mg / mL magnetic beads) to the centrifuge tube. Place the centrifuge tube on the magnetic separation rack for 2 min, and carefully remove the supernatant. Add 1mL of blocking agent, mix thoroughly with a vortex mixer, then place the centrifuge tube on the magnetic separation rack for 2min (or longer), and carefully remove the supernatant. Repeat the blocking step a total of 3 times. Add 1 mL of blocking agent again, and mix well using a vortex mixer. Add 100 μg of coating antibody and vortex to mix well. Use a 360° rotary mixer to incubate at room temperature for 3 h. Place the centrifuge t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com