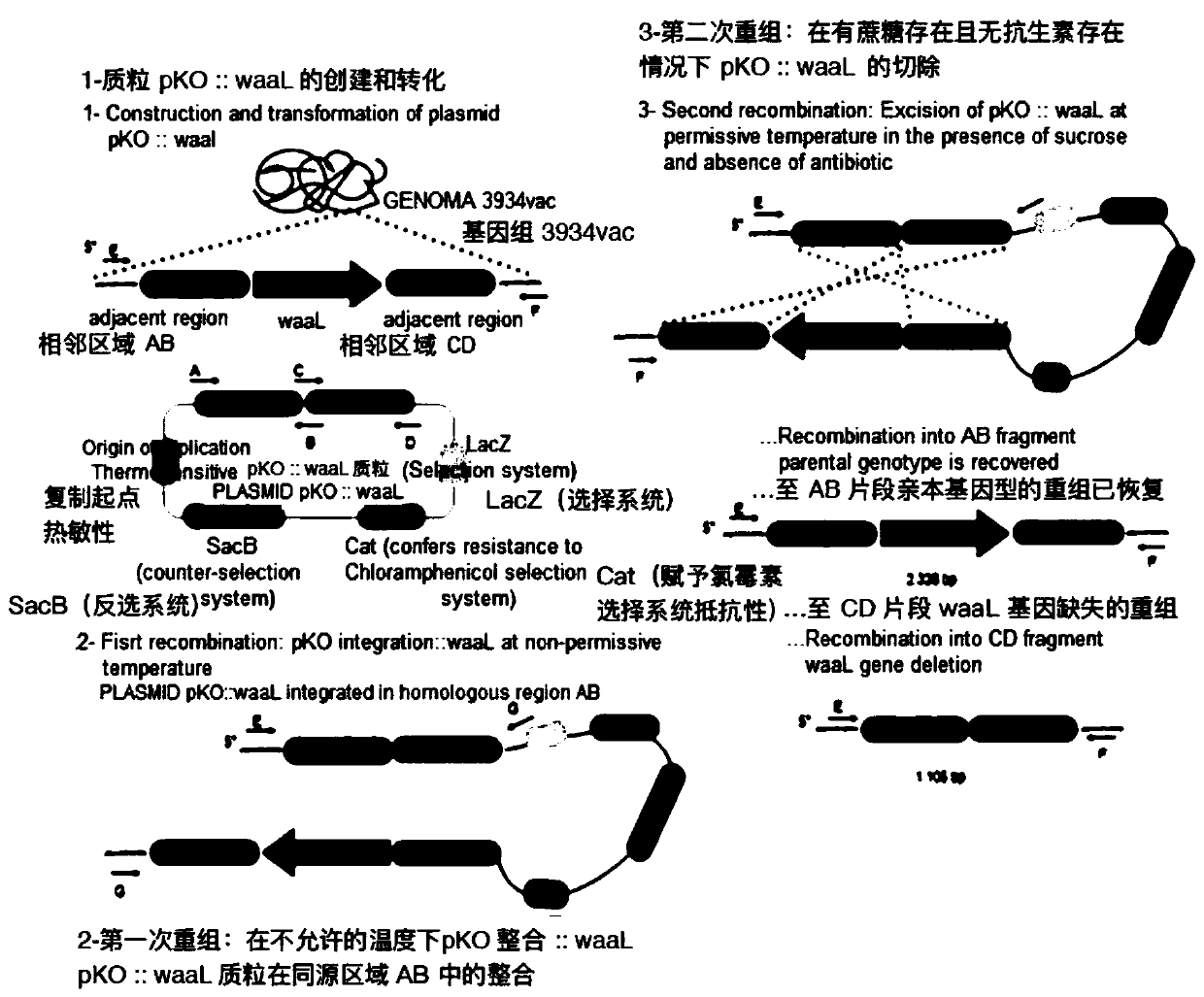
Production of rough-type salmonella enteritidis and genetic modifications thereof for use as avian vaccine
A Salmonella Enteritidis, gene technology, applied in veterinary vaccines, vaccines, genetic engineering and other directions, can solve problems such as decreased immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
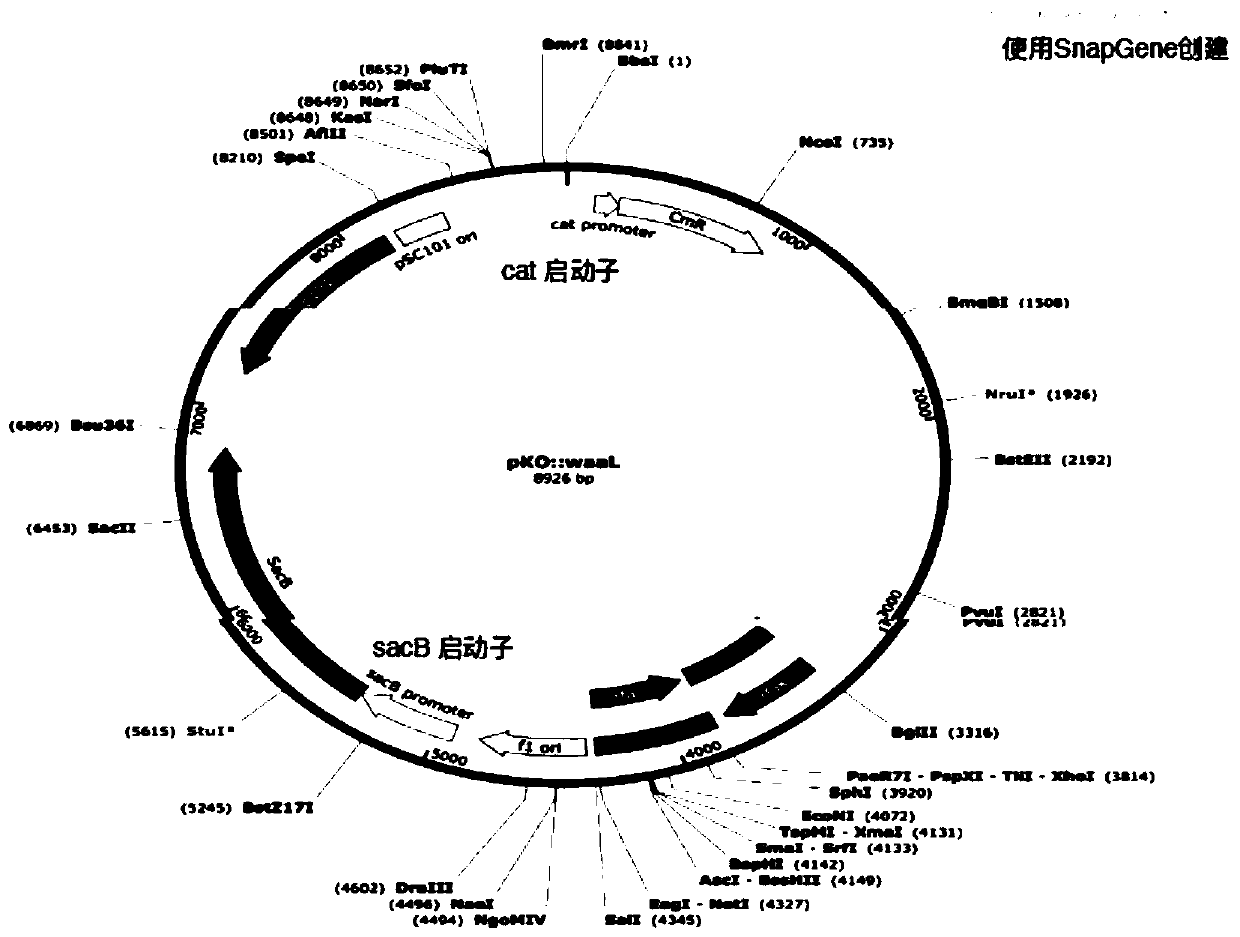
Image
Examples
Embodiment approach
[0119] Preferred Embodiments of the Invention: Examples
[0120] The following examples are provided herein to illustrate the nature of the invention. These examples are for illustrative purposes only and should not be construed as limitations of the invention.
example 1
[0121] Example 1: Expression of fiber antigens in Salmonella Enteritidis 3934Vac strain by immunoblotting.
[0122] protein extraction
[0123] We placed 1 colony in 5 mL of LB medium for ClyA fibers with an insertion in the chromosome. Incubate overnight at 37 °C at 200 rpm.
[0124] We centrifuged at 110000rpm for 5 minutes, then added lysis buffer 1 (Tris HCL 10Mm, EDTA 5mM, NaCl 50mM) + protease inhibitors 40μL (3g / mL) + 50μL lysozyme (10mg / mL), and SDS of each sample Buffer (50 μL Easy Buffer 2x + 50 μL Urea 8M + 5 μL B-Mercaptoethanol 50 μL sample), homogenize and heat at 100°C for 5 minutes, then place on ice for 5 minutes, take 20 μL for loading. Use 0.1Amps for each gel at 1:30.
[0125] A clyA-fiberFAdV-His protein band was observed in the results of Western blot. Markers used; ladder P7709V (175KDa) on the left, ab 116029 (245KDa) on the right ( Figure 10 ).
[0126] Also, when using two positive His+ controls, but with the initials A and B by weight, A is ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com