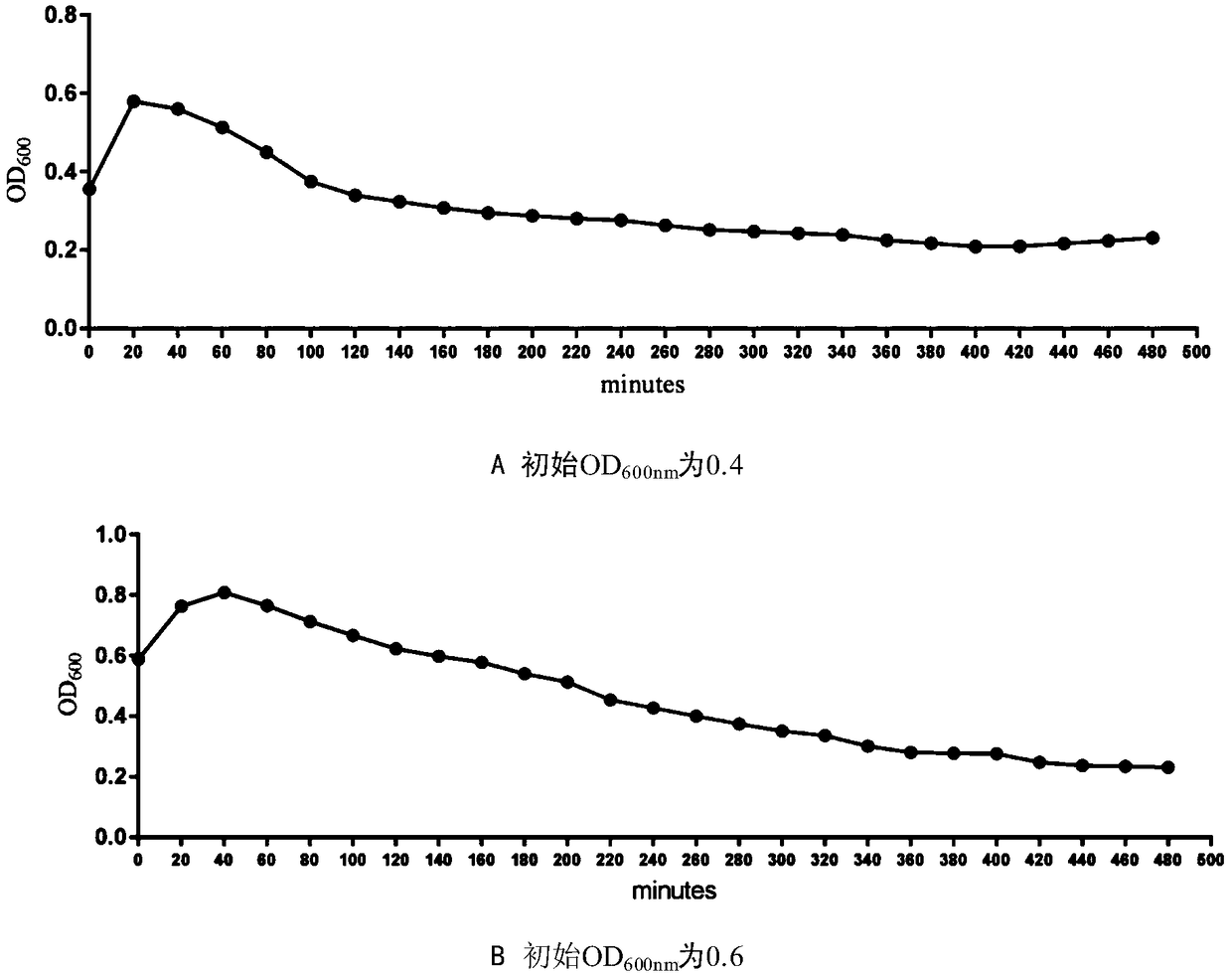
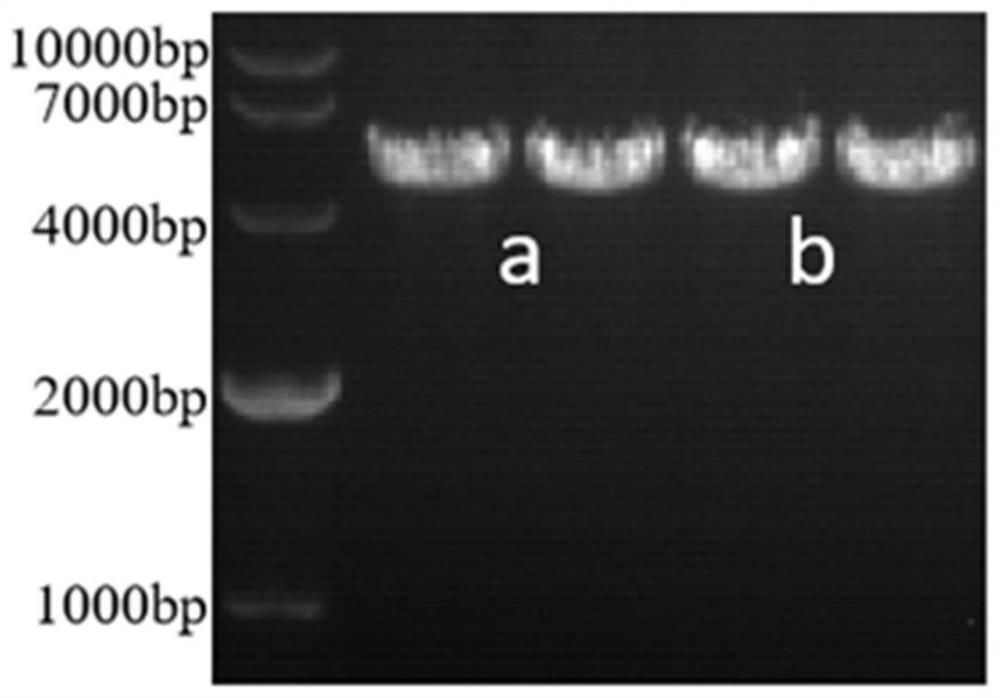
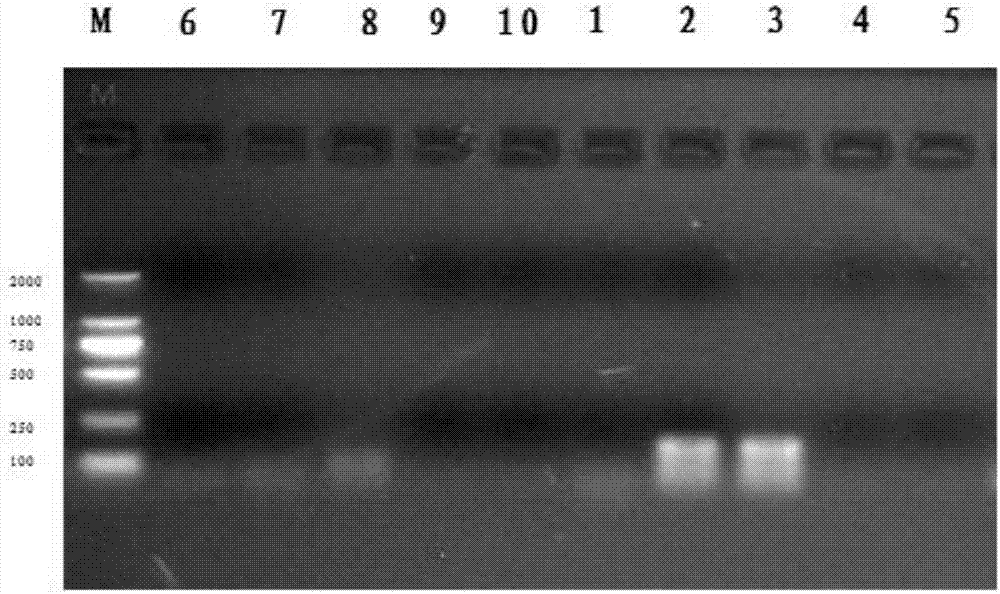
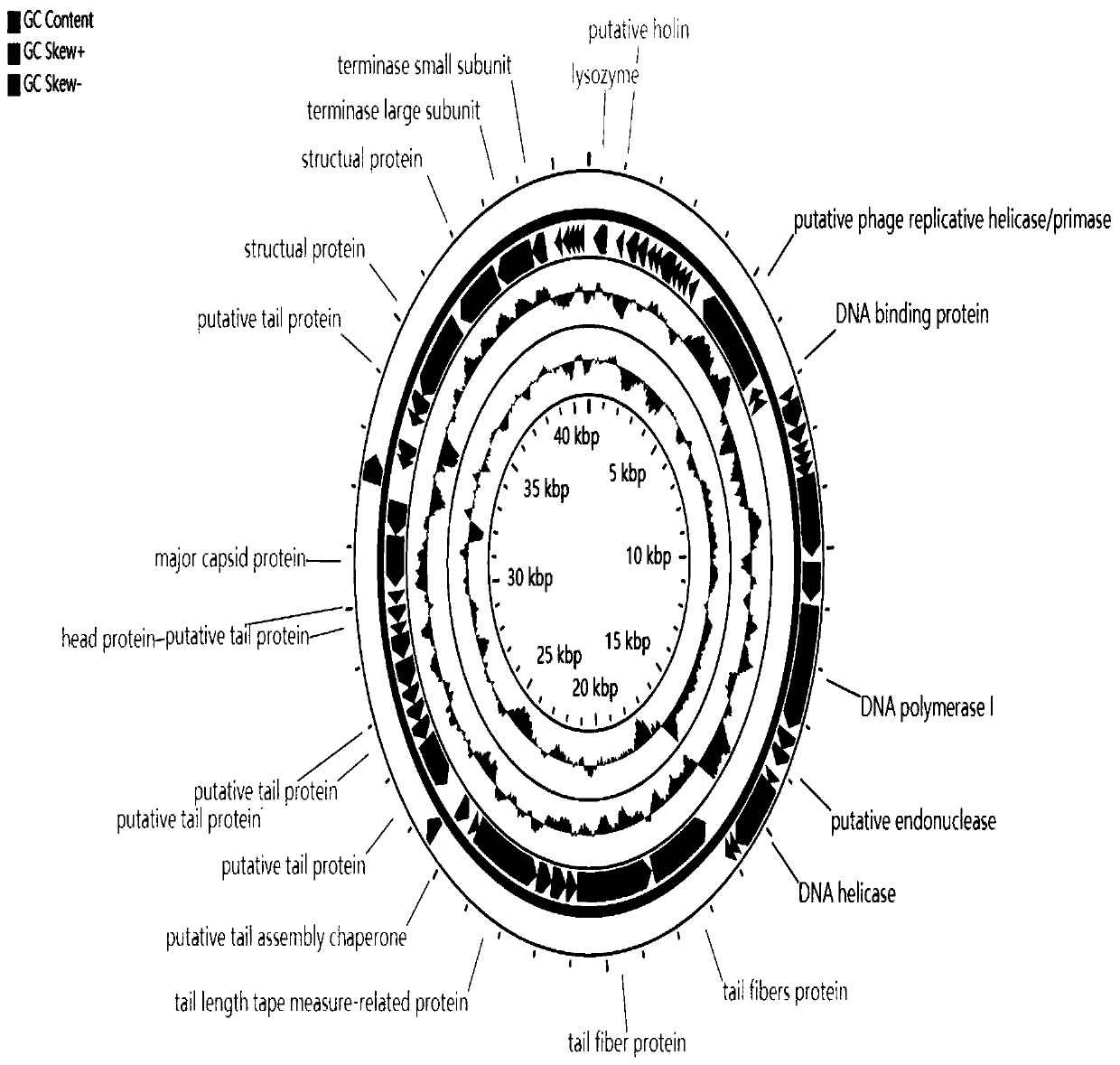
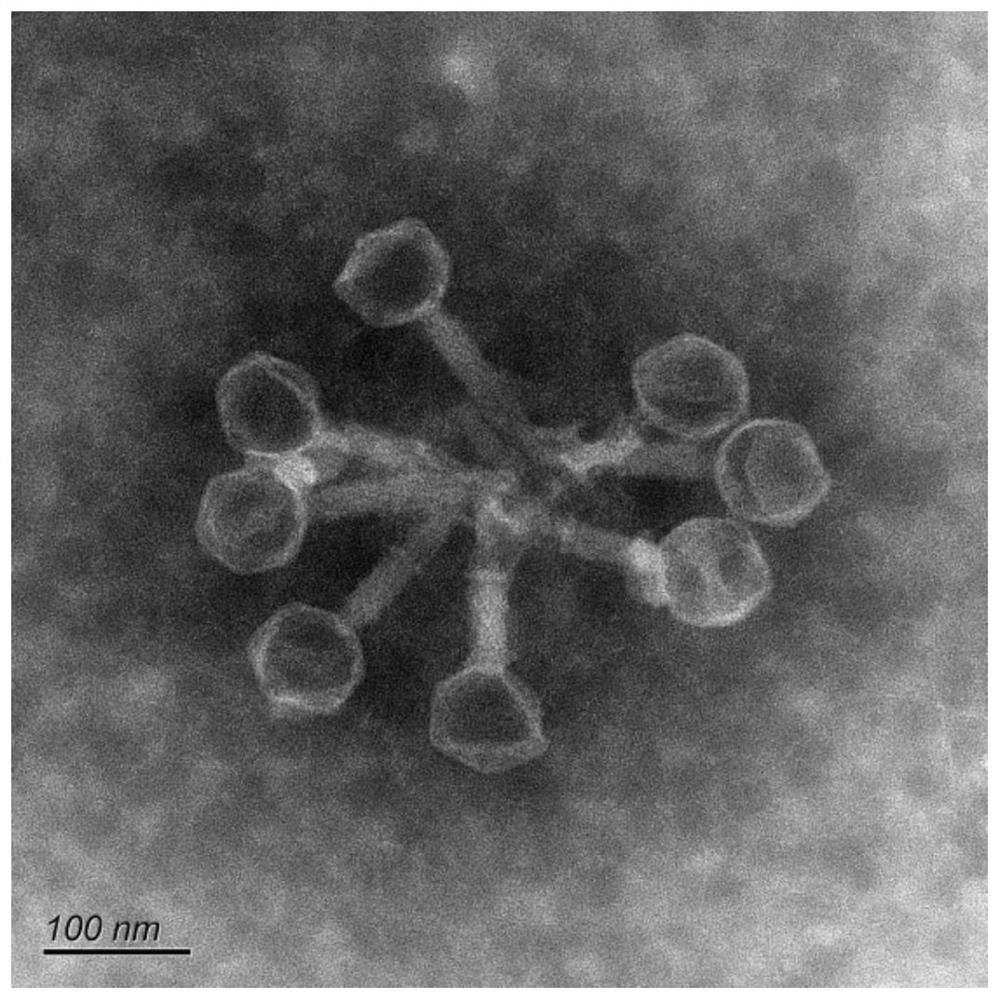
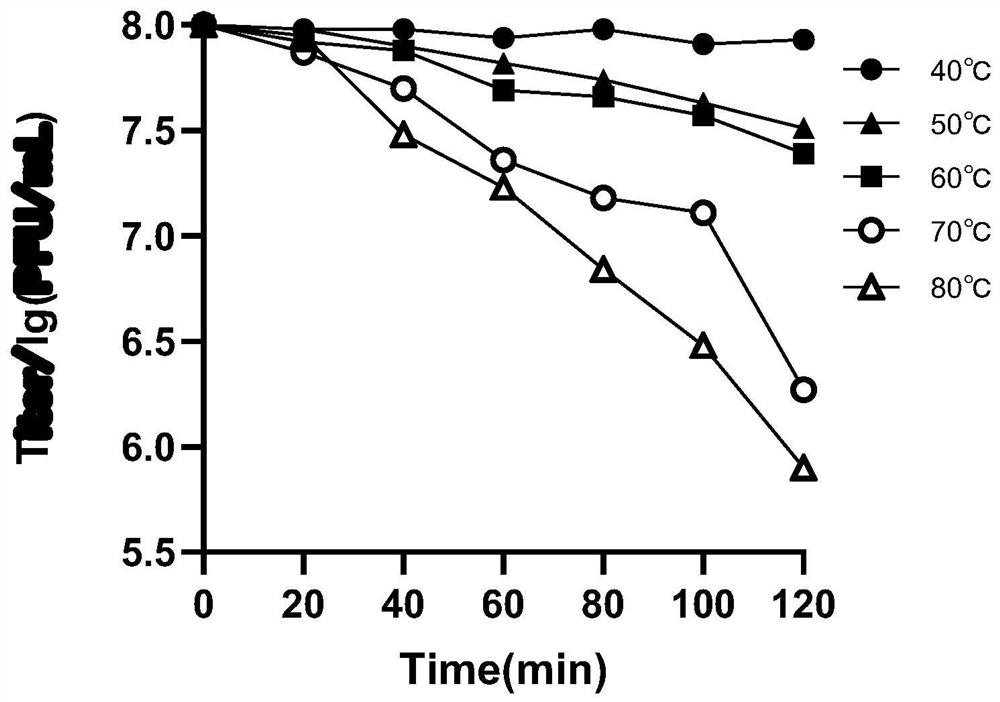
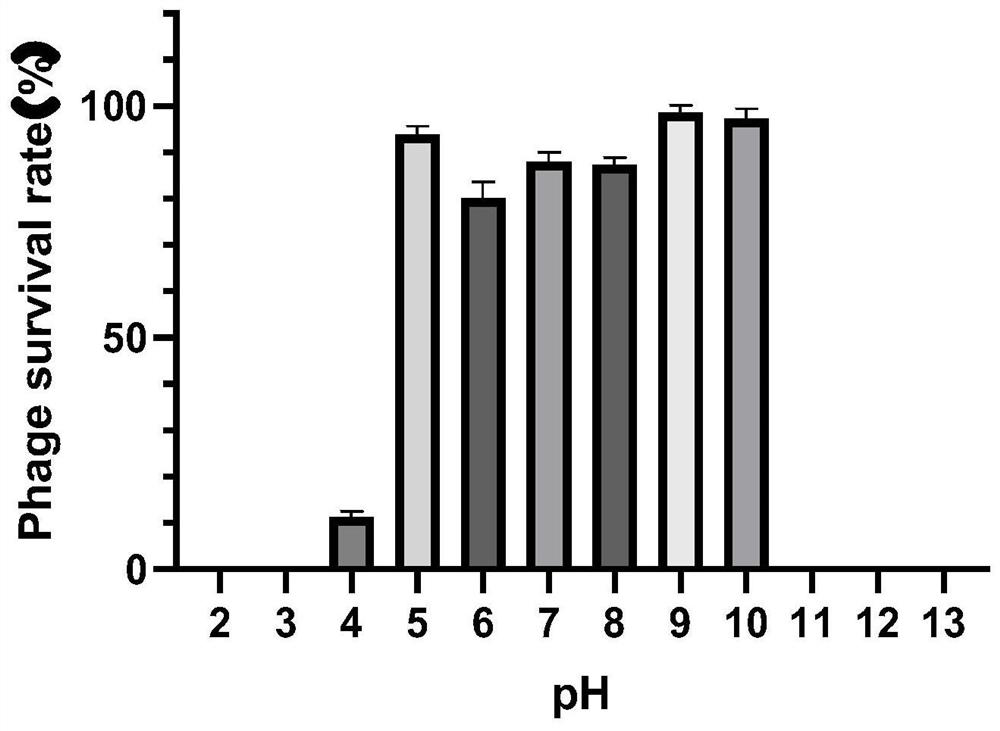
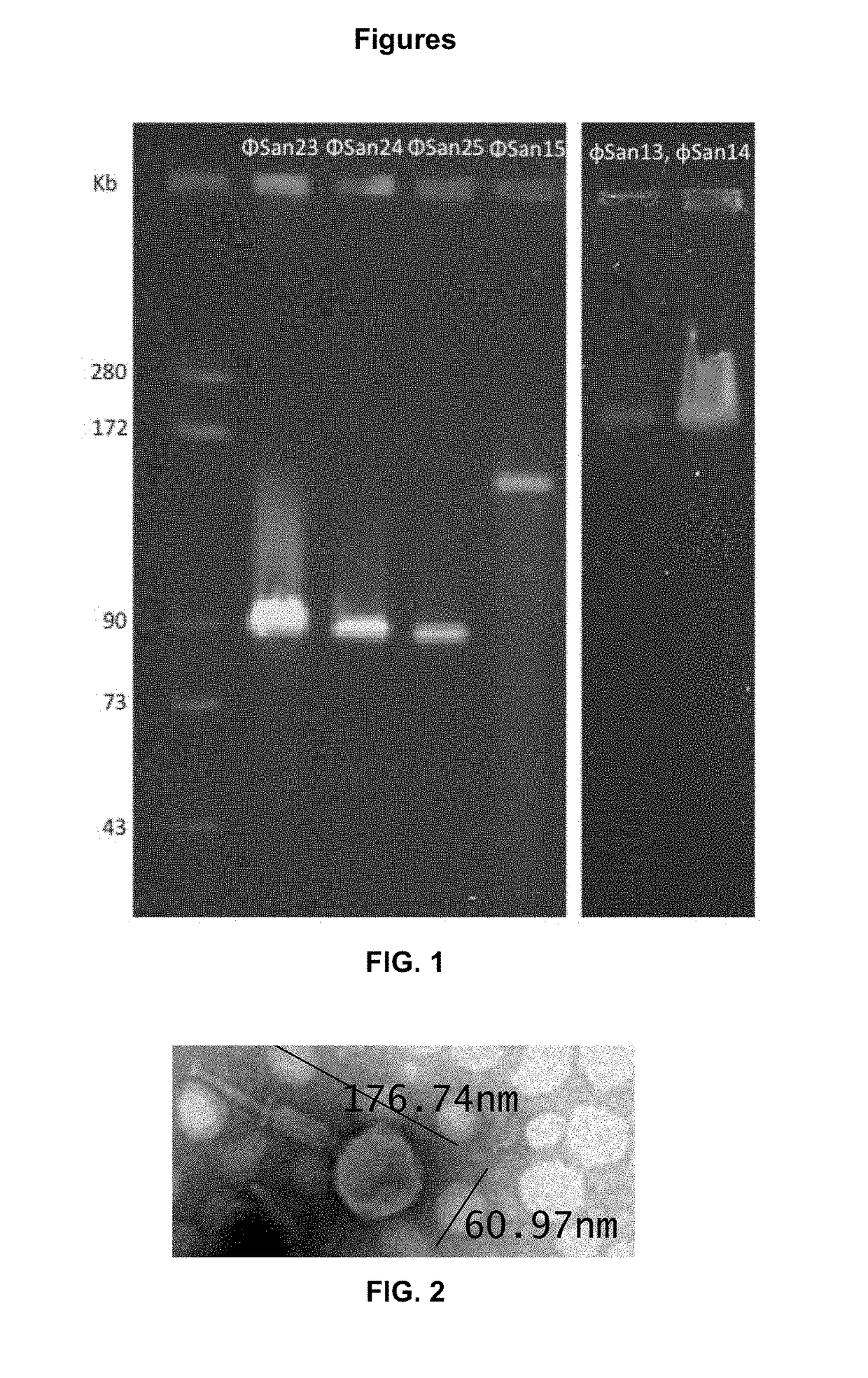
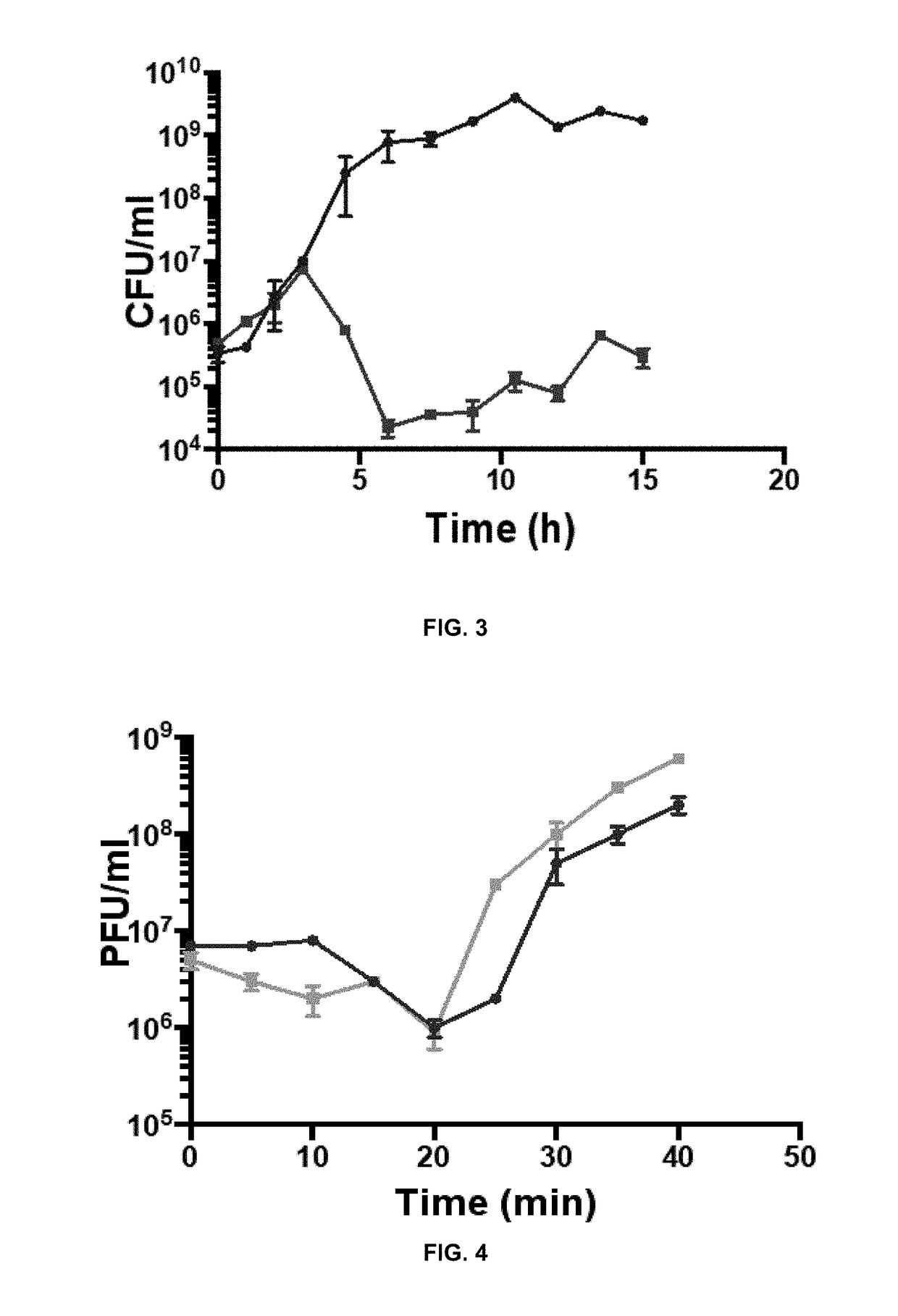
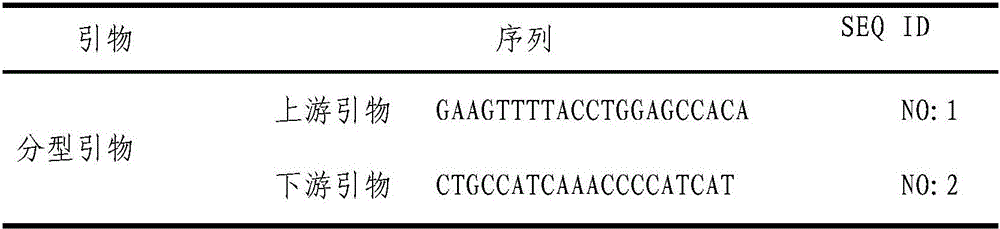
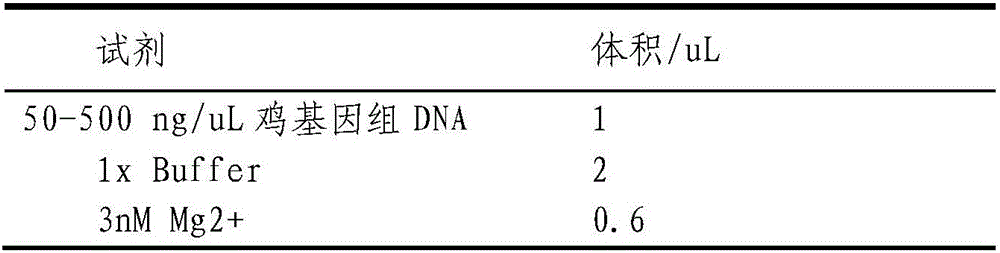
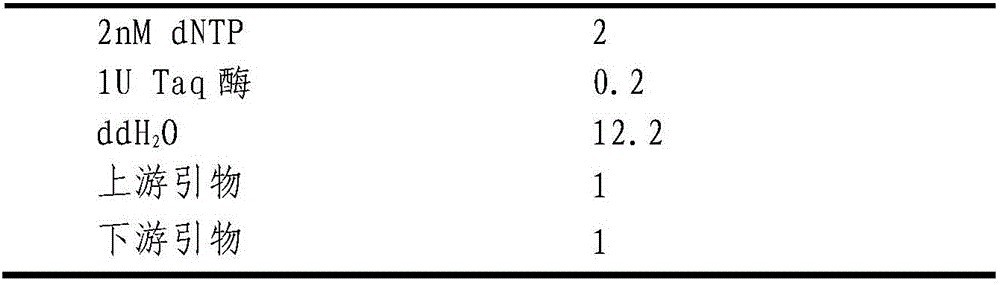
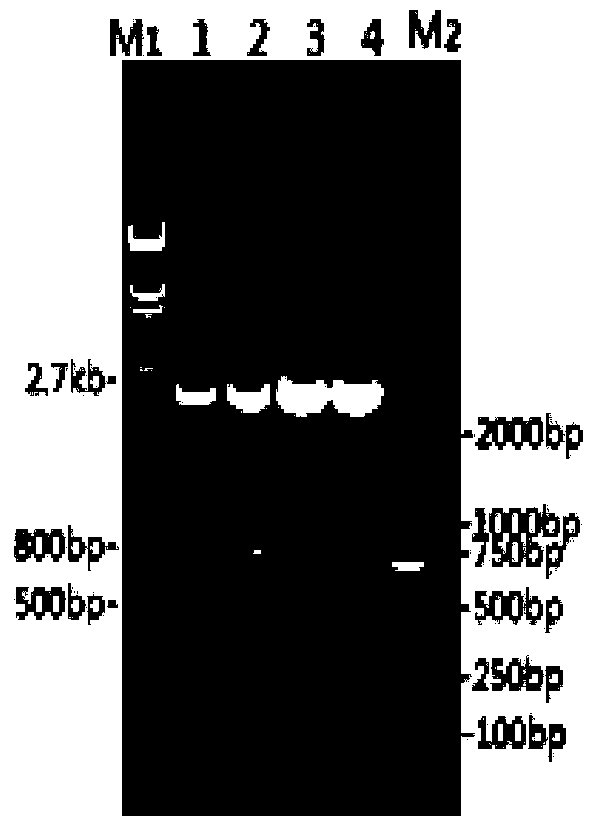Patents
Literature
53 results about "Salmonella enterica serovar enteritidis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
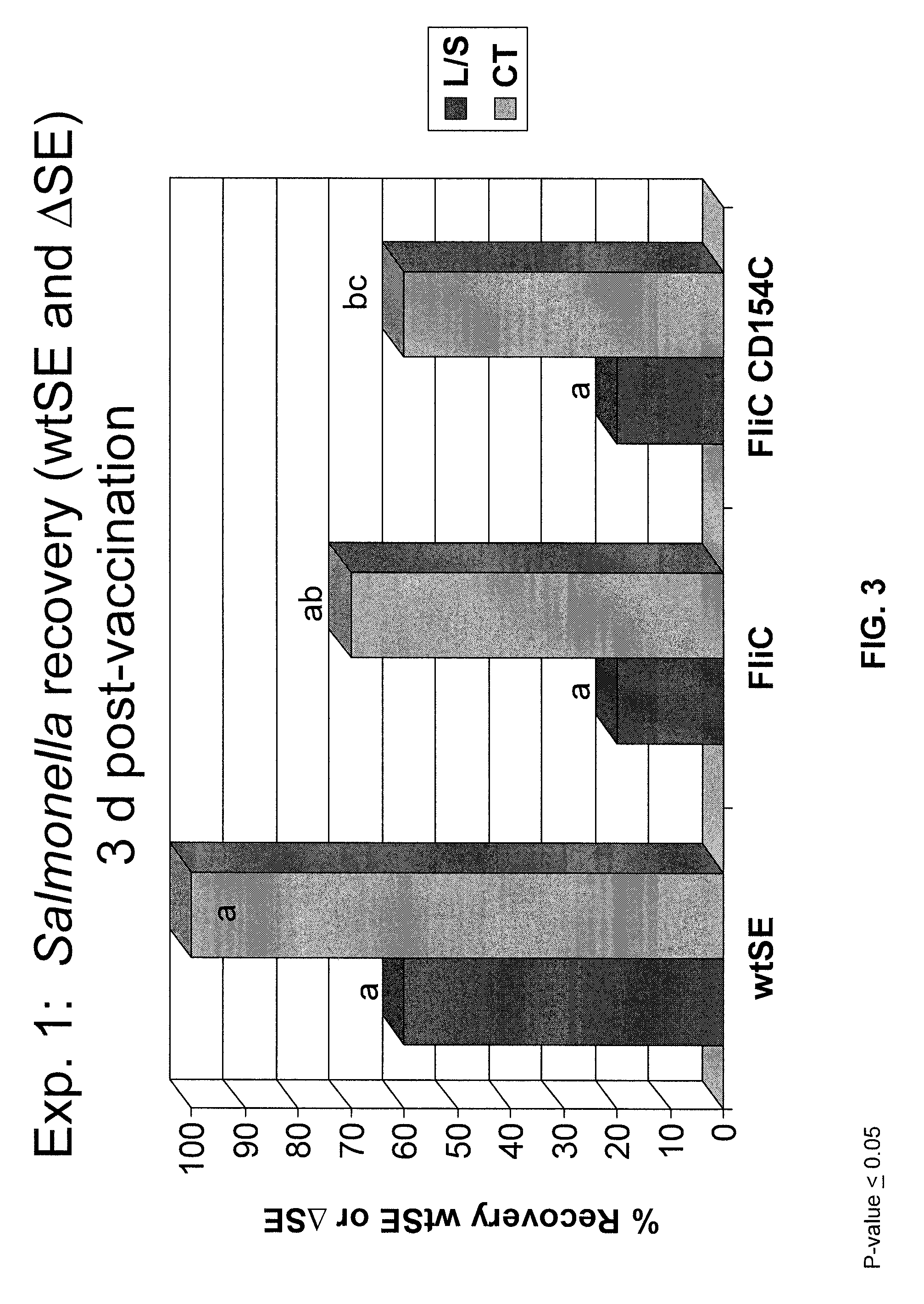
Compositions and methods of enhancing immune responses to flagellated bacterium
ActiveUS9125854B2Enhance immune responseReduce morbidityAntibacterial agentsBacterial antigen ingredientsSalmonella enteritidisImmunology
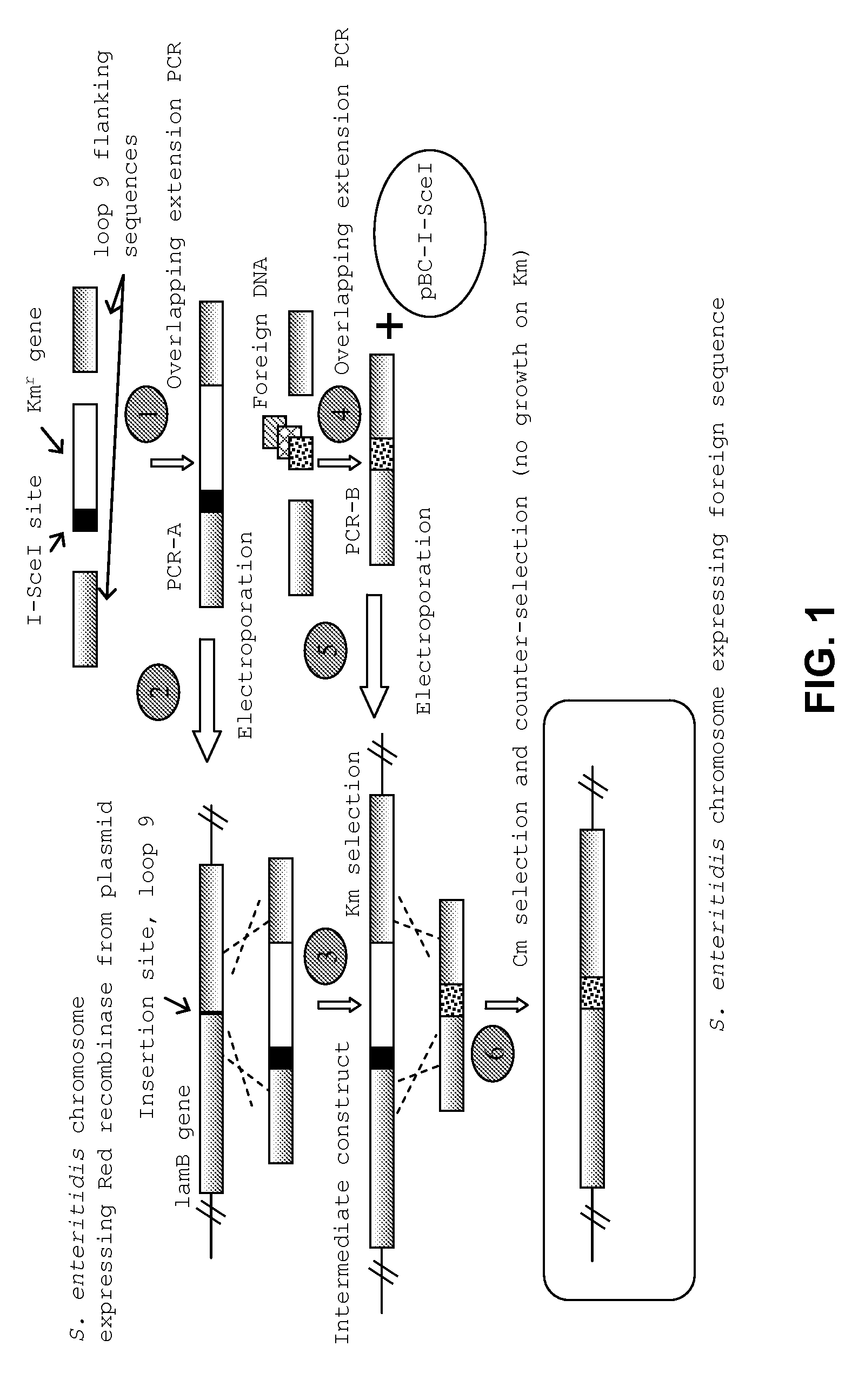
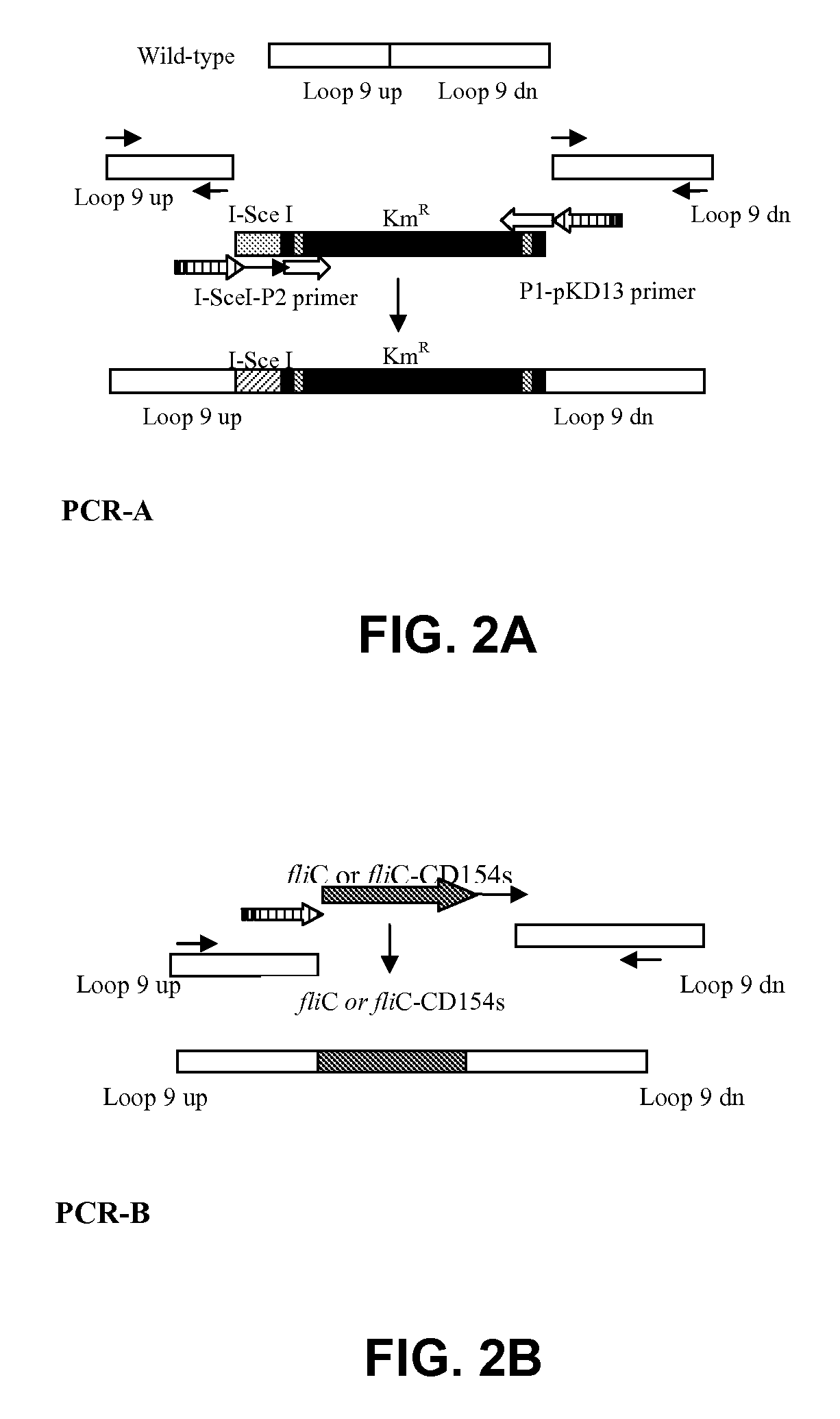
Vaccines comprising fliC and CD 154 polypeptides and Salmonella enteritidis vaccine vectors comprising fliC polypeptides are provided. Also provided arc methods of enhancing an immune response against flagellated bacteria and methods of reducing morbidity associated with infection with flagellated bacteria.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
Saccharomyces cerevisiae and culture thereof and application thereof to feed
InactiveCN108085261AGrow fastImprove growth performanceFungiAnimal feeding stuffDiseaseEscherichia coli
The invention discloses saccharomyces cerevisiae 5-1 which is collected in the Guangdong Microbial Culture Collection Center, with the collection number of GDMCC No: 60243. The invention further discloses a fermentation solution, a culture and a culture dilution prepared from the saccharomyces cerevisiae 5-1. A metabolite of the saccharomyces cerevisiae 5-1 has a very strong inhibitory effect on pathogenic bacteria (escherichia coli, staphylococcus aureus and salmonella enteritidis); when the saccharomyces cerevisiae 5-1 is used as a feed additive and is added into feed for feeding animals, the disease incidence of the animals can be effectively reduced, the growth performance of the animals can be improved, and the intestinal microflora of the animals can be significantly improved, so that the saccharomyces cerevisiae 5-1 is of a great significance in development of the breeding industry.
Owner:潘韵
Novel bacteriophage and antibacterial composition comprising same
InactiveCN102149816AImprove acid resistanceImprove heat resistanceAntibacterial agentsMammal material medical ingredientsDisinfectantFood poisoning
The present invention relates to a novel bacteriophage, and more specifically to a bacteriophage that can specifically destroy one or more Salmonella spp. selected from a group consisting of Salmonella enteritidis, Salmonella typhimurium, Salmonella gallinarum and Salmonella pullorum. The present invention further relates to a composition containing the bacteriophage as an active ingredient for the prevention or treatment of infectious diseases such as salmonellosis and salmonella foodborne intoxication induced by Salmonella enteritidis or Salmonella typhimurium, fowl typhoid induced by Salmonella gallinarum, pullorum induced by Salmonella pullorum, etc. Moreover, the present invention relates to animal feeds, drinking water, detergent and disinfectant containing the bacteriophage as an inactive ingredient.
Owner:CJ CHEILJEDANG CORP
Salmonella enteritidis double knockout attenuated mutant and preparation as well as application thereof
The invention discloses a salmonella enteritidis double knockout attenuated mutant and preparation as well as application thereof. The salmonella enteritidis double knockout attenuated mutant is obtained by knocking out crp gene and spiC gene of salmonella enteritidis C50041. The invention further discloses a preparation method as well as application of the salmonella enteritidis double knockout attenuated mutant. Further attenuation of the salmonella enteritidis attenuated mutant is realized. A foundation is laid for researching salmonella enteritidis attenuated live vaccines and live vector vaccines.
Owner:YANGZHOU UNIV
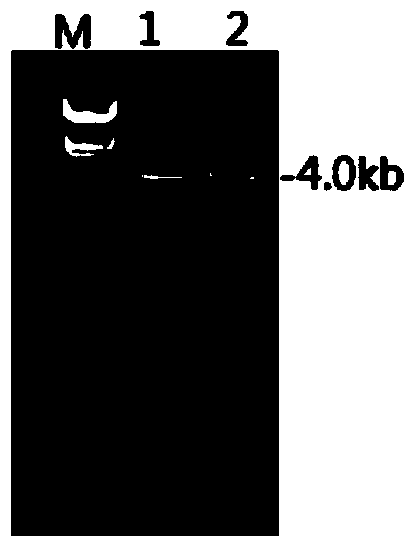
Salmonella enteritidis nucleic acid standard sample as well as building method and application thereof
InactiveCN102719424ASolve the shortage situationFix stability issuesMicrobiological testing/measurementMicroorganism based processesBiotechnologySocial benefits
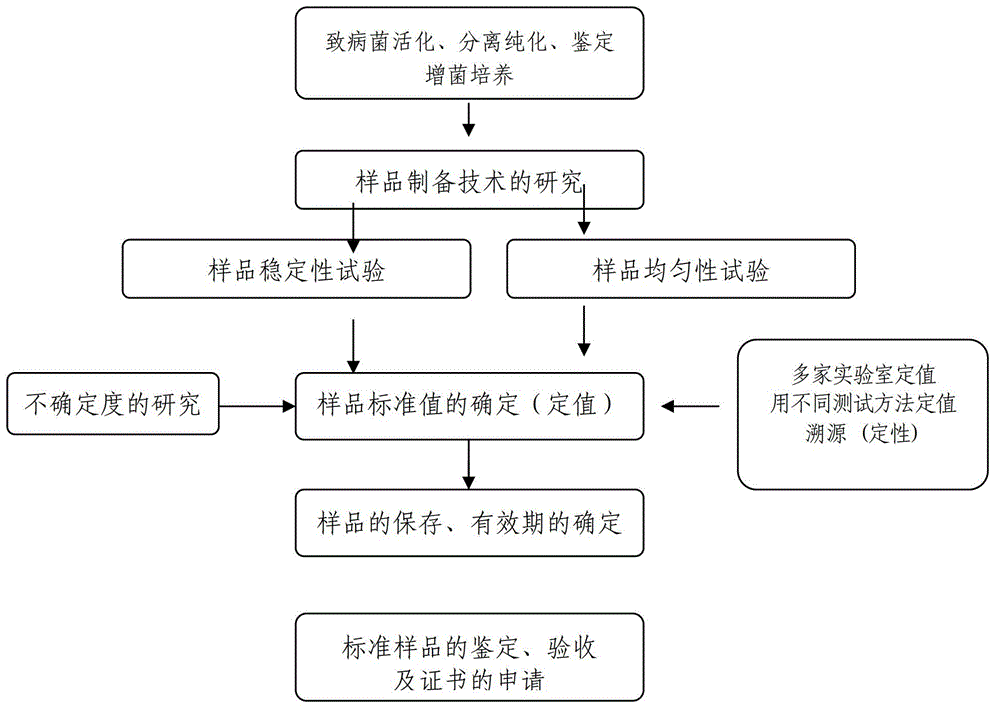
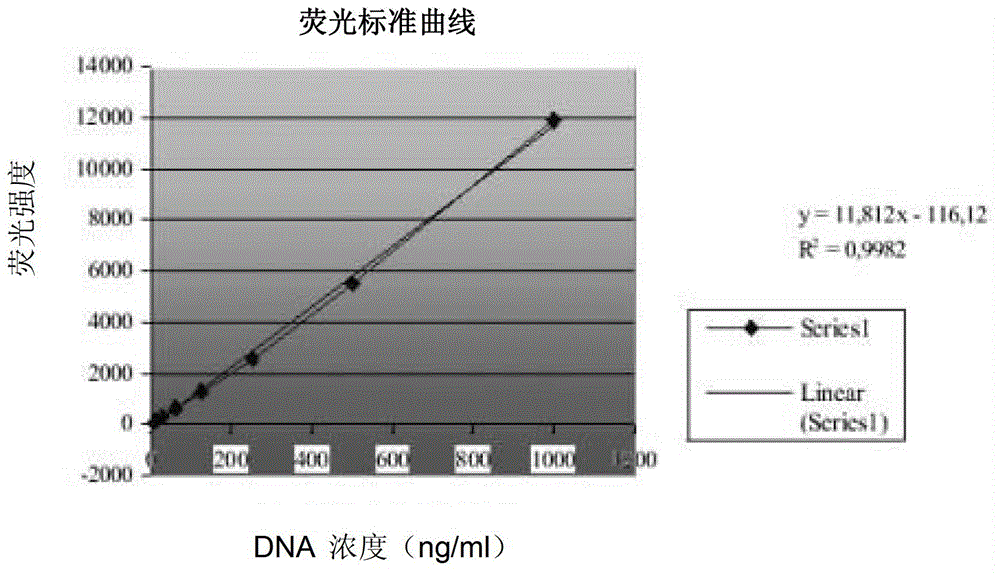
The invention discloses a Salmonella enteritidis nucleic acid standard sample as well as a building method and application thereof. The building method comprises the steps: strain culture, identification, preparation of a nucleic acid standard sample, drying of the nucleic acid standard sample, uniformity and stability inspection, qualitative assay, rating of the nucleic acid standard sample and other operations to obtain the Salmonella enteritidis nucleic acid standard sample. According to the invention, the condition that the pathogen nucleic acid standard sample is deficient in domestic food is solved, has important practical significance in realizing preparation technology of the pathogen nucleic acid standard sample in food and stability guarantee technology, positively developing the research and development of pathogen molecular biological detection standard sample in domestic food, and filling up the blank of the measuring field, and has a broad market in developing molecular biological detection working of pathogen in the food and food pathogen nucleic acid standard sample, and has greater economic benefits and social benefits.
Owner:曹际娟 +3
Novel bacteriophage and antibacterial composition comprising the same
Disclosed herein are is a novel bacteriophage which has specific bactericidal activity against one or more Salmonella bacteria selected from the group consisting of Salmonella Enteritidis, Salmonella Typhimurium, Salmonella Gallinarum, and Salmonella Pullorum without affecting beneficial bacteria. Disclosed are also compositions, animal feeds or drinking water, cleaners and sanitizers for preventing and treating the infectious diseases caused by Salmonella Enteritidis, Salmonella Typhimurium, Salmonella Gallinarum or Salmonella Pullorum including salmonellosis, Salmonella food poisoning, Fowl Typhoid, and Pullorum disease or for controlling the salmonella bacteria.
Owner:CJ CHEILJEDANG CORP
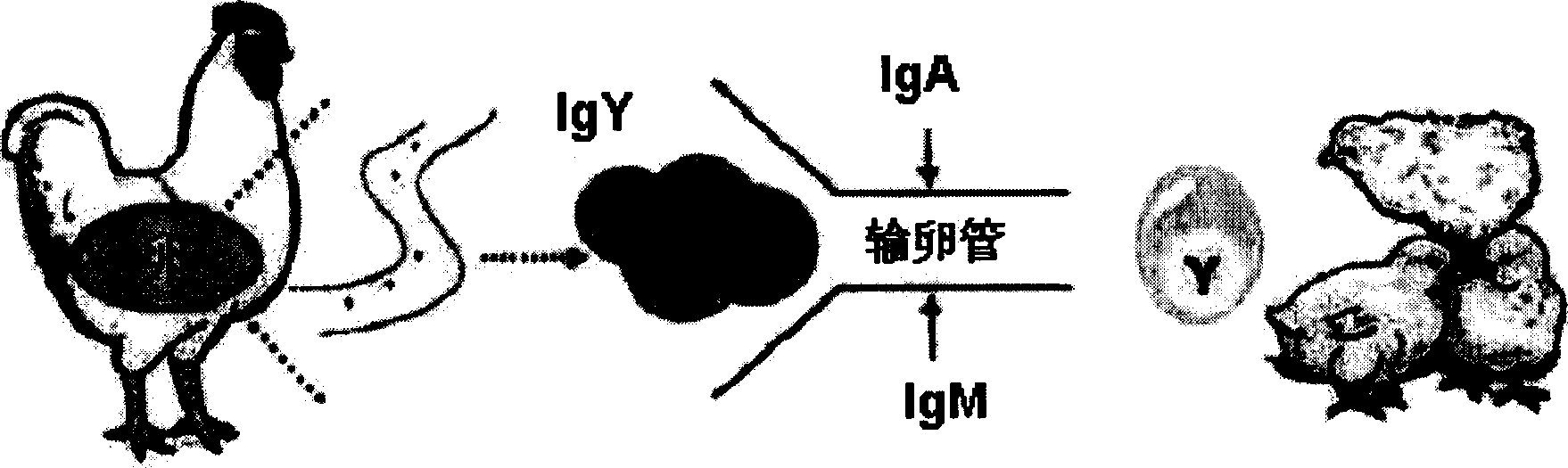
Method for production of egg containing anti-pathogenic bacteria specific antibodies (IgY) and yogurt and ice cream containing IgY
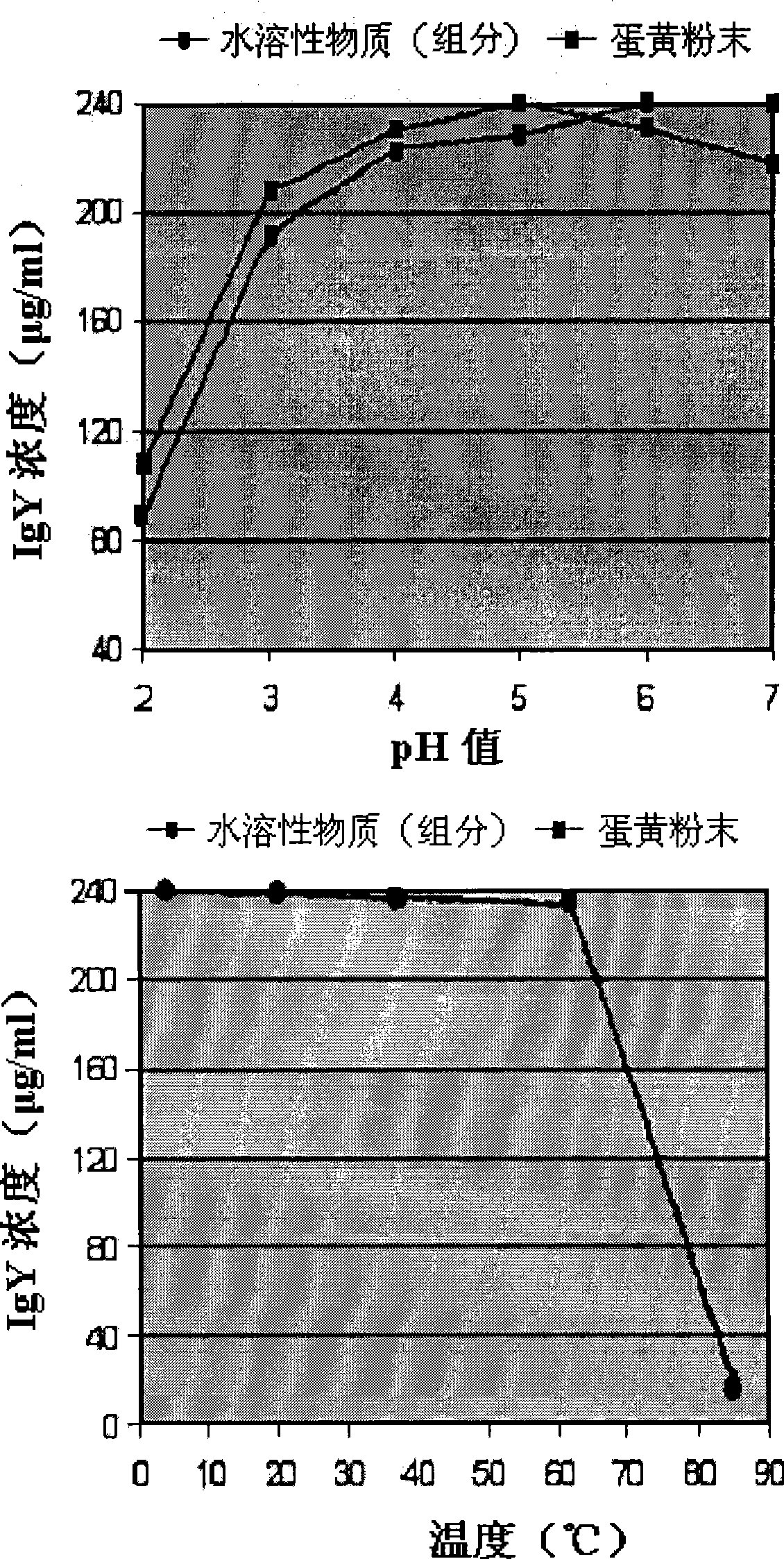
InactiveCN1411378AAvoid secondary infectionPrevent enteritisMilk preparationEgg immunoglobulinsBiotechnologyYolk
Owner:EGG BIOTECH
Growth inhibitory composition against pathogenic bacteria of meat based food stuff comprising igy
InactiveCN101400701AFunctionalProgramme-controlled sewing machinesEgg immunoglobulinsBiotechnologyYolk
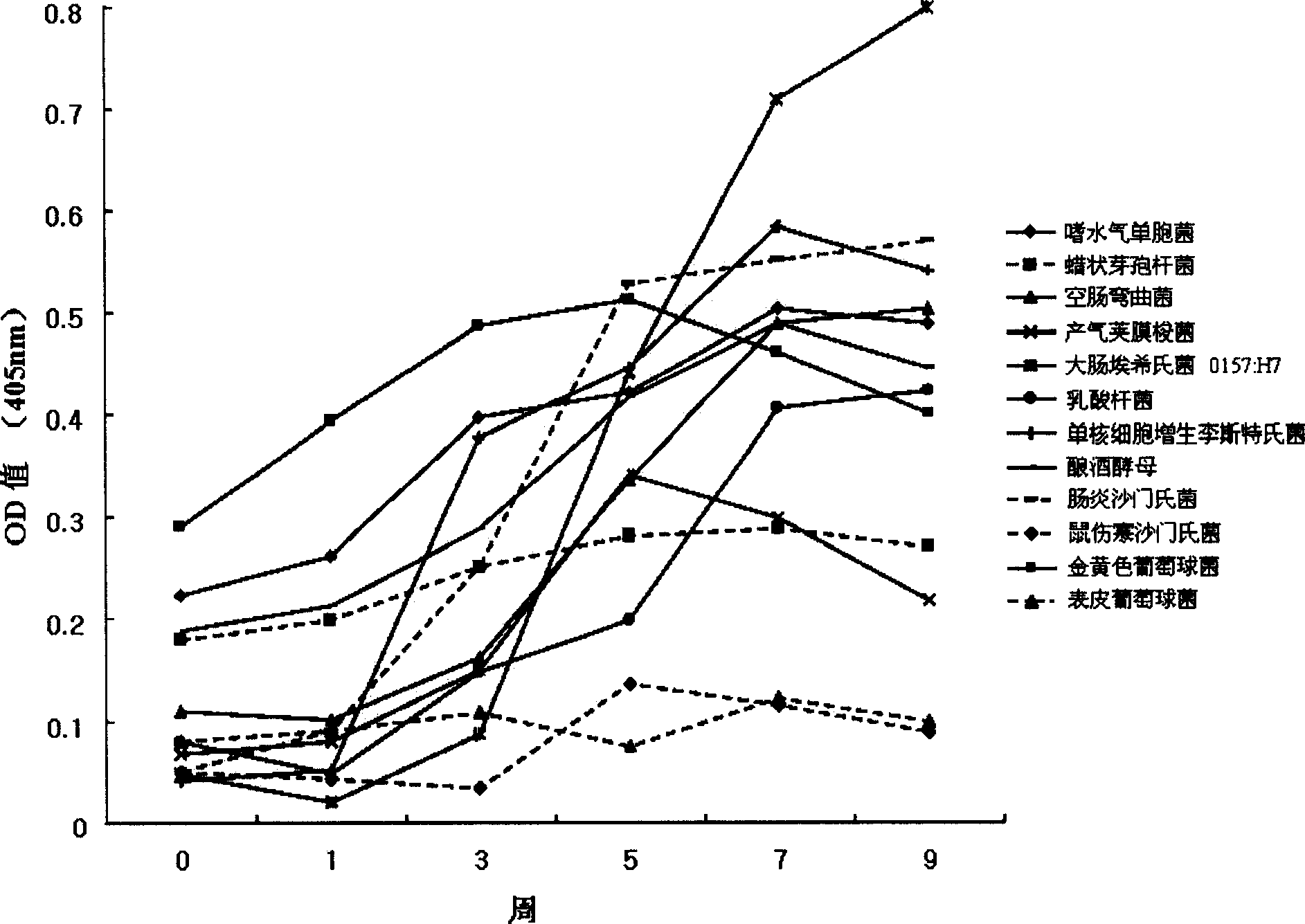
The invention relates to growth-inhibitory composition against pathogenic bacteria of meat based food stuff comprising IgY. The IgY is a specific immunoglobulin derived from yolk of egg. 12 representative bacteria and other microbes deteriorating quality of process meat products that the IgY of the invention targets include Aeromonas hydrophila, Bacillus cereus, which comprises Aeromonas hydrophil, Bacillus cereus, Camphlobacter jejuni, Clotridium perfringens, 0157:H7 (Escherichia col, 0157:H7), Lactobacillus, Listeria monocytogens, Saccharomyces cerevisiae, Salmonella enteritidis, Salmonella typhimurium, Staphylococcus aureus, Staphylococcus epidermidis. The 12 representative bacteria antigen forms are respectively injected the interior of chicks to obtain the IgY of the 12 antigens.
Owner:CJ CHEILJEDANG CORP +1
Chicken salmonella enteritidis infection resistance molecular marker Wnt7b detection method and application thereof
ActiveCN106434922AAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesAgricultural scienceGenotype
The invention relates to the technical field of gene engineering, in particular to a chicken salmonella enteritidis infection resistance molecular marker Wnt7b detection method and application thereof. A chicken Wnt7b gene rs313644723 site genotyping specific primer and genotyping PCR (polymerase chain reaction) conditions matched with the primer are provided herein, salmonella enteritidis infection resistance related site genotypes can be accurately detected by using the primer and the method, and theoretical basis and scientific basis are provided for screening and disease-resistant breeding of chicken salmonella enteritidis infection resistant individuals.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Application of pyroptosis-associated protein GSDMD (Gasdermin-D) for preparing bacterial ghost vaccine
ActiveCN109303916AAvoid damageInfection controlAntibacterial agentsPeptide/protein ingredientsHumoral immune reactionOrganism
The invention discloses application of pyroptosis-associated protein GSDMD (Gasdermin-D) for preparing a bacterial ghost vaccine. A bacteriolysis plasmid containing a pyroptosis-associated protein GSDMD coding gene is constructed and is converted into salmonella enteritidis; under the induction of gum sugar, bacteria are split to successfully obtain a salmonella novel bacterial ghost vaccine. An experiment proves that GSDMD mediated bacteriolysis has an extremely long persistent period, and a splitting rate is 99.9985% or more. After a novel bacterial ghost immune mouse prepared by the invention is adopted, an organism can be stimulated to generate powerful humoral immunity and cellular immunity; protective immunity response is induced; an experiment animal can be protected so as to resistsalmonella infection. The salmonella bacterial ghost vaccine prepared by the invention has a good immune protection effect.
Owner:HARBIN WEIKE BIOTECH DEV
Method for the detection of Salmonella enterica serovar Enteritidis
Described herein is the identification of a novel Salmonella enterica serovar Enteritidis locus that serves as a marker for DNA-based identification of this bacterium. In addition, three primer pairs derived from this locus that may be used in a nucleotide detection method to detect the presence of the bacterium are also disclosed herein.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
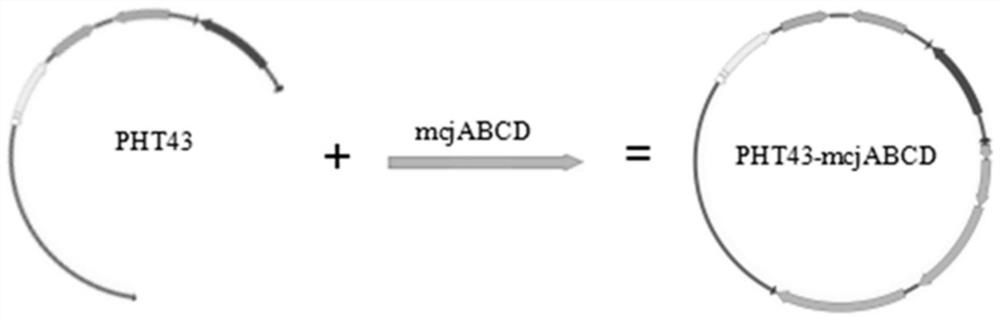
Bacillus subtilis engineering bacterium for expressing MccJ25 as well as construction method and application of bacillus subtilis engineering bacterium
ActiveCN114574412AEconomical and quick way to useAchieve colonizationAntibacterial agentsBacteriaBiotechnologyAntibiosis
The invention discloses a bacillus subtilis engineering bacterium for expressing MccJ25 as well as a construction method and application of the bacillus subtilis engineering bacterium. The engineering bacterium is constructed by cloning a mcjABCD sequence which is modified by lasso peptide MccJ25 to bacillus subtilis. Wherein the sequence of the mcjABCD is as shown in SEQ ID NO. 1. The constructed bacillus subtilis engineering bacteria can successfully express the MccJ25, and the bacillus subtilis belongs to food-grade probiotics and can avoid the influence of endotoxin, so that the bacillus subtilis engineering bacteria can be used as probiotics for producing the MccJ25; meanwhile, the engineering bacterium also has a relatively strong inhibition effect on salmonella enteritidis and the like, and can be used in the aspect of antibacterial products so as to improve the application value of MccJ25.
Owner:SOUTH CHINA AGRI UNIV
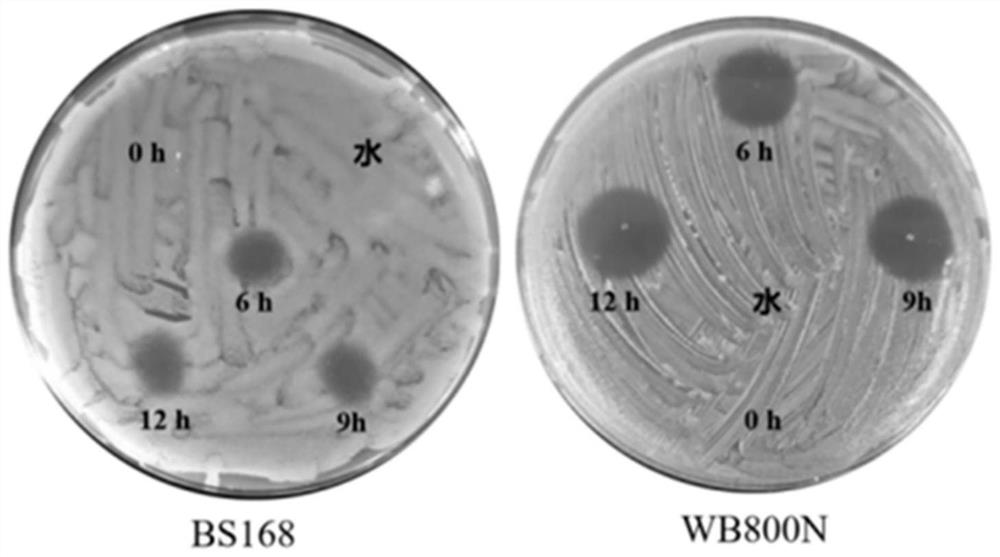
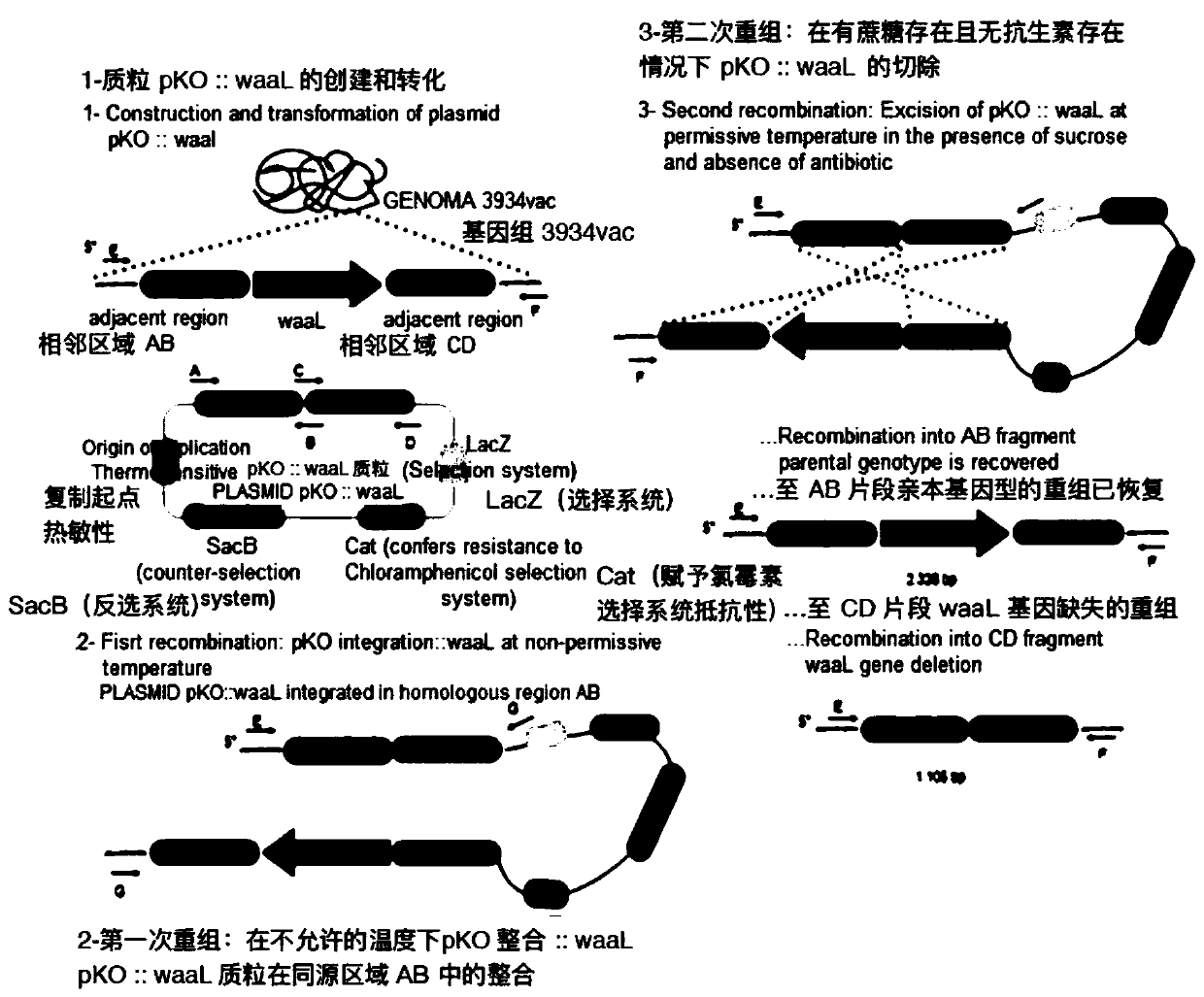
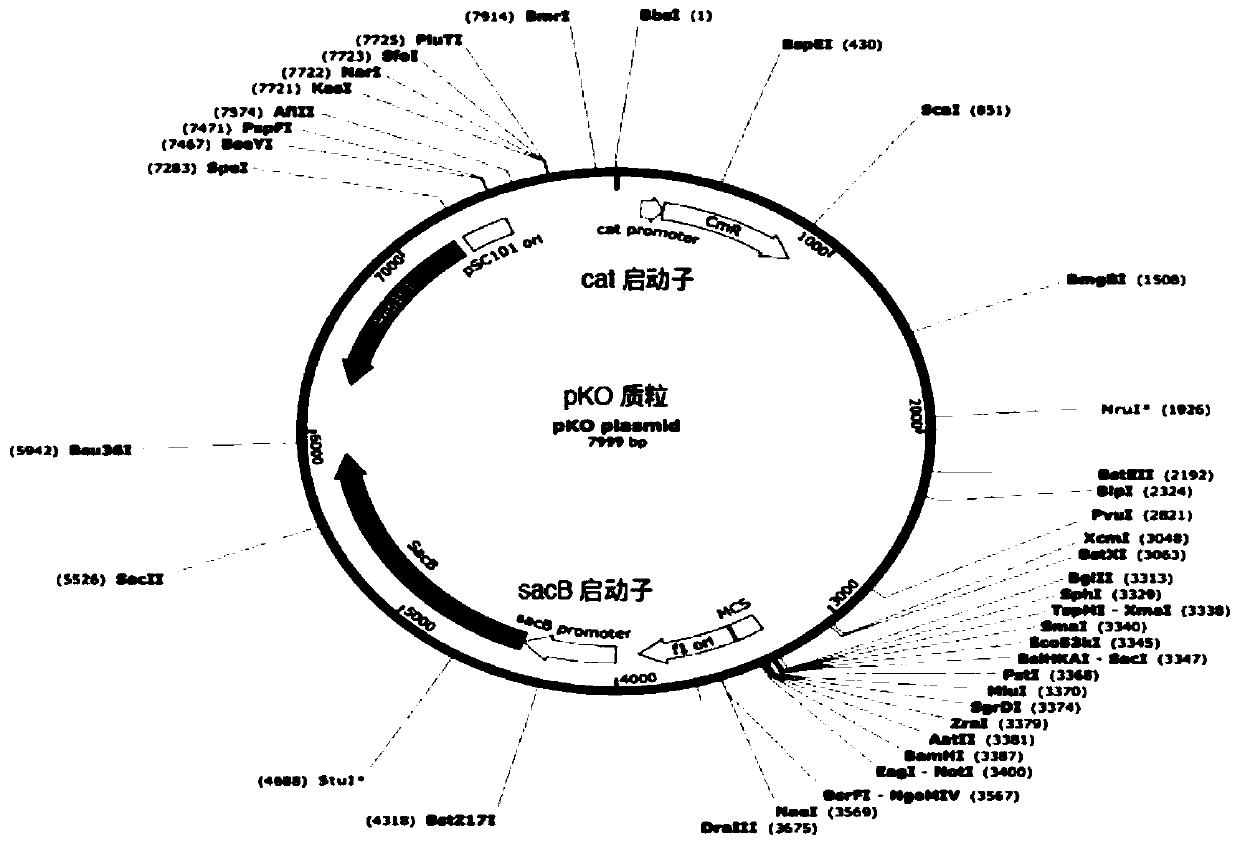
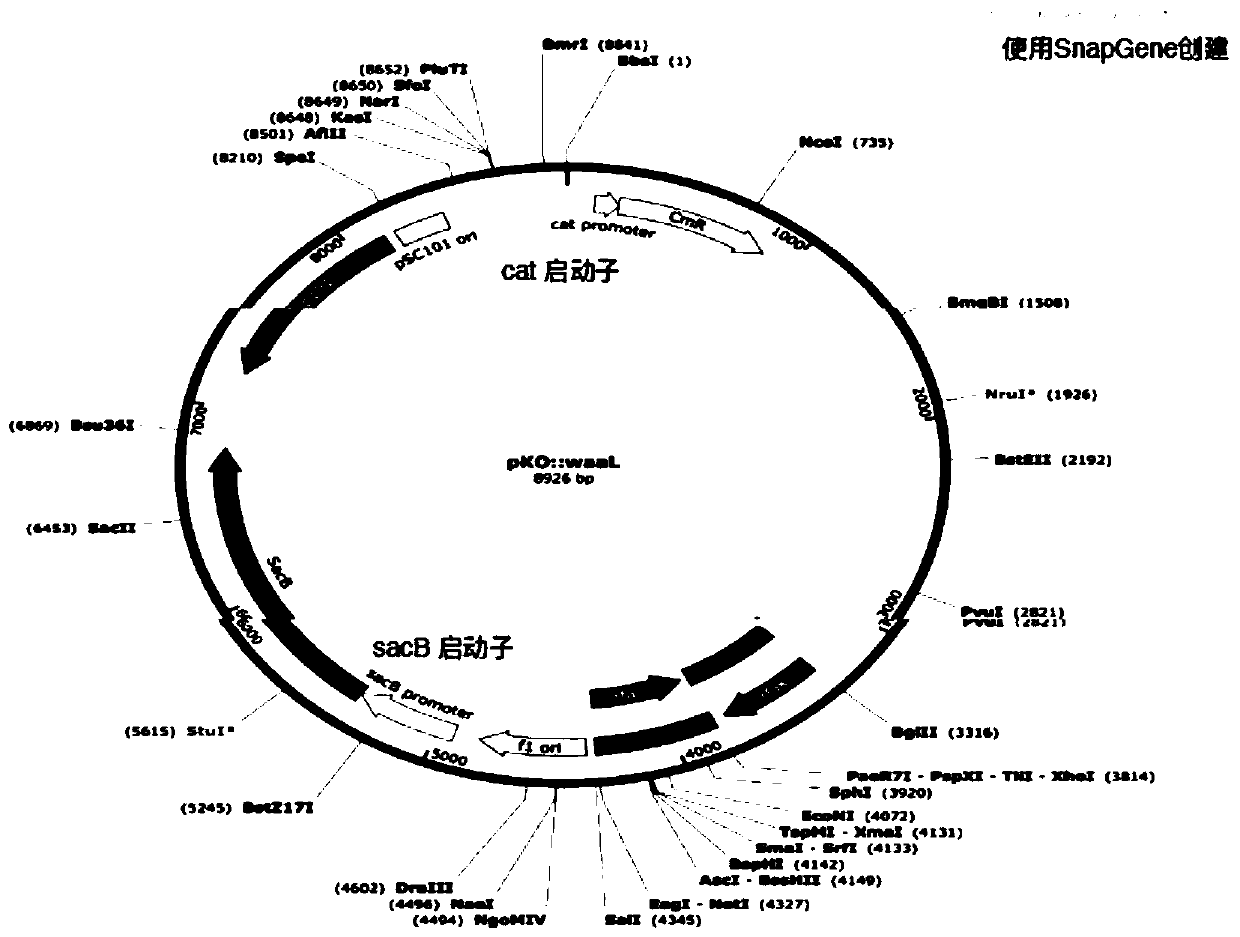
Production of rough-type salmonella enteritidis and genetic modifications thereof for use as avian vaccine
The present invention relates to a strain of salmonella enteritidis 3934vac from which the waaL gene has been deleted in order to obtain a rough phenotype (3934vac DwaaL), as well as to the method forobtaining the same and to the oligos used, with a view to reducing toxicity and maintaining immunogenicity for the use of the same as a vaccine. The present invention also relates to a strain of thesalmonella enteritidis 3934vac DwaaL, i.e. of the rough type, which has been modified to express the fibre gene of type-1 avian adenovirus, as well as to the method for obtaining the strain of the salmonella enteritidis 3034 vac DwaaL which expresses a fibre gene of Ava-I. The invention further comprises the development of a novel avian vaccine against the AvA-I virus, which is live, recombinant,effective and harmless and was developed by using a process involving inserting and integrating AVa-I fibre genes into the chromosome of a non-pathogenic attenuated strain of the salmonella enteritidis bacteria.
Owner:兽医制药股份公司
Salmonella bacteriophage with wide lysis spectrum and high temperature resistance and application thereof
ActiveCN113430173AStrong cracking effectNo side effectsAntibacterial agentsBiocideDiseaseTherapeutic effect
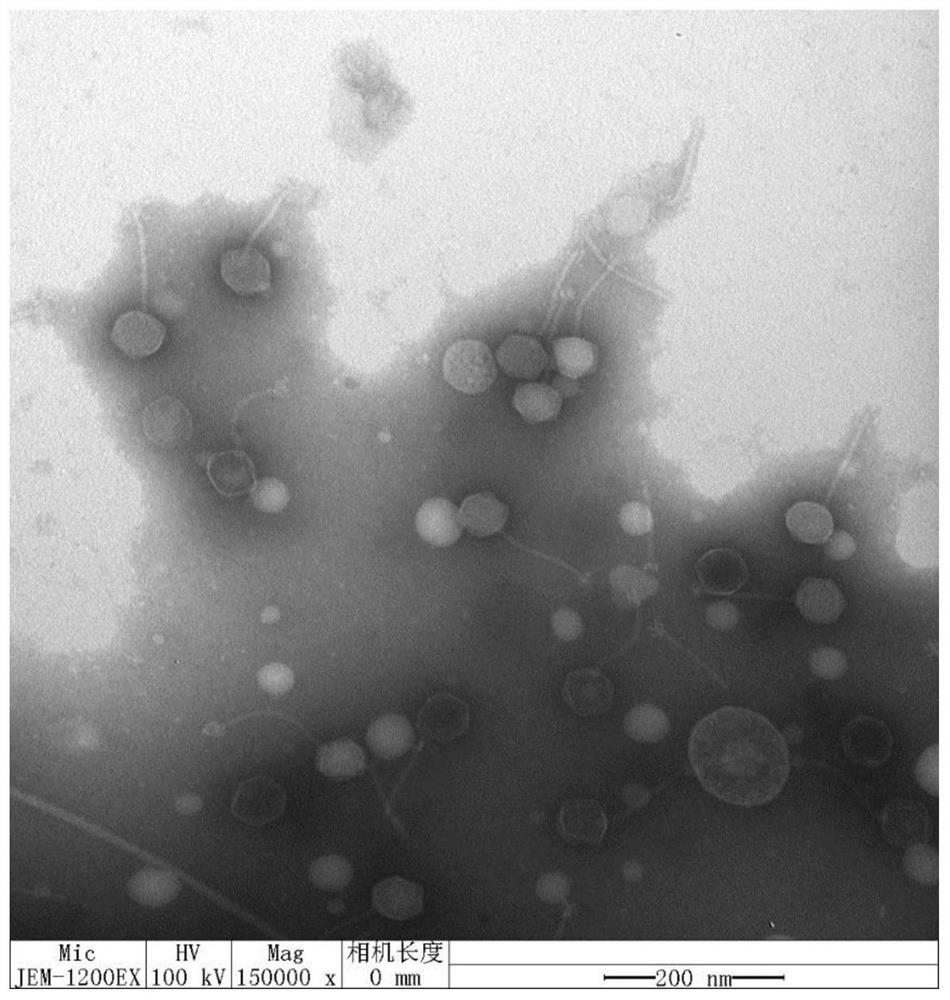
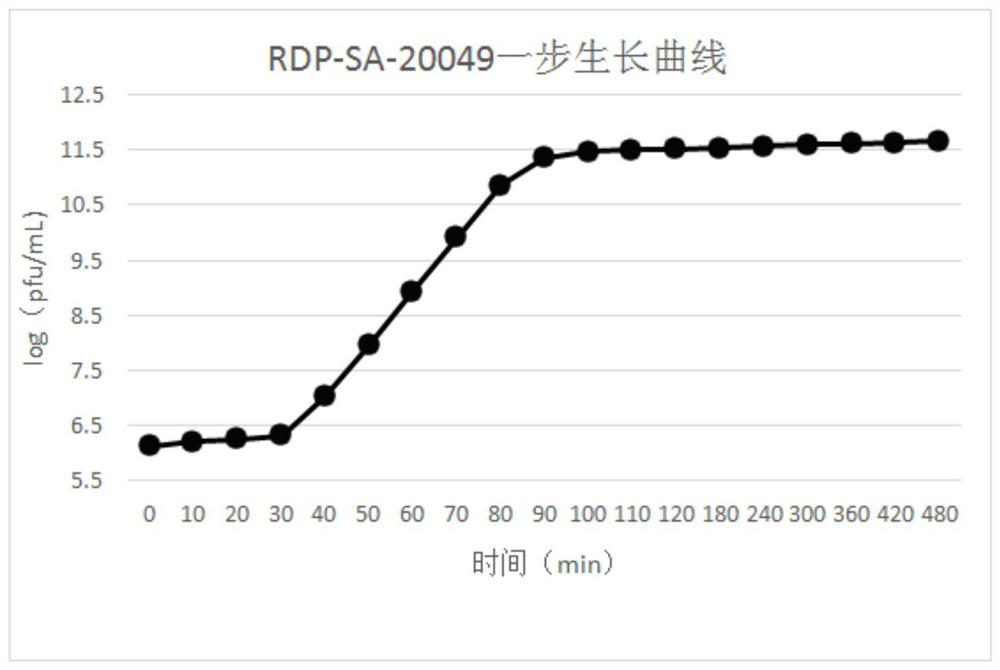
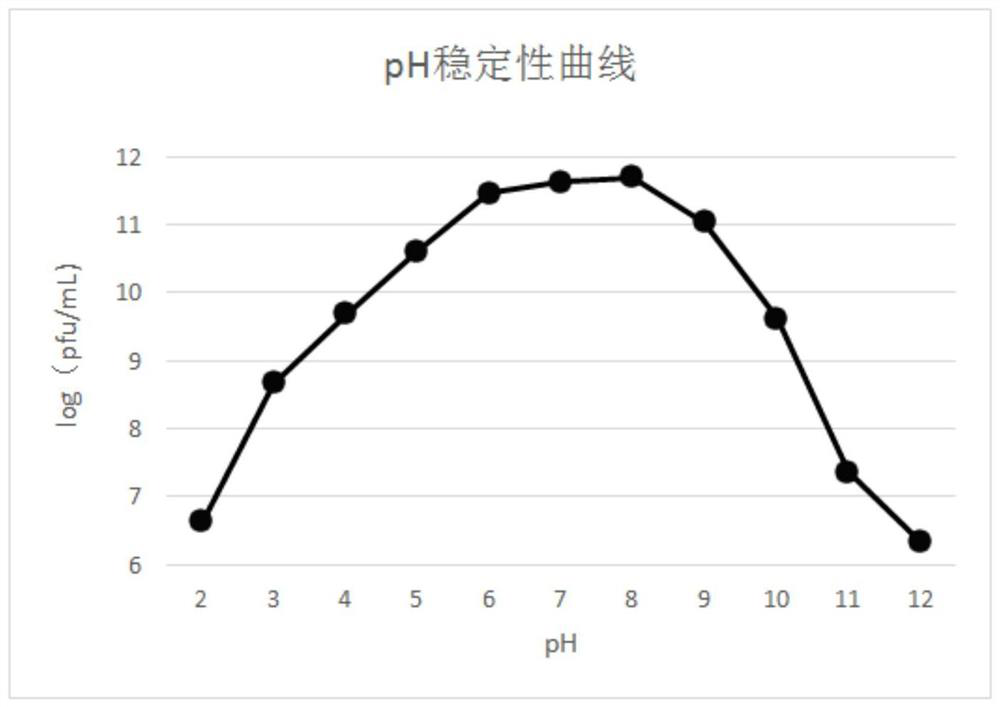
The invention discloses a salmonella bacteriophage with a wide lysis spectrum and high temperature resistance. The preservation number is CGMCC No.21995. After whole genes are sequenced, the salmonella bacteriophage does not have virulence genes and lyogenic genes. The salmonella bacteriophage RDP-SA-20049 acts for 60 min at the high temperature of 90 DEG C, the titer is kept at 1.0 * 10 < 7 > pfu / mL or above, and the salmonella bacteriophage RDP-SA-20049 has high biological activity and good high temperature resistance. The salmonella bacteriophage can be used for lysing pathogenic bacteria including 5 strains of salmonella typhimurium, 4 strains of salmonella pullorum, 1 strain of salmonella paratyphi B, 1 strain of salmonella typhimurium, 7 strains of salmonella enteritidis and salmonella BS-20036, and has the characteristic of high cracking rate, the cracking rate is up to 99%, the cracking performance is stronger, and the salmonella bacteriophage has good advantages in treatment of salmonella infection diseases, and is high in titer and good in prevention and treatment effect.
Owner:RECOM QINGDAO BIOTECH CO LTD
Salmonella broad-spectrum lyase with in-vitro lytic activity and application thereof
ActiveCN111471670AHigh in vitro antibacterial activityImprove sterilization efficiencyAntibacterial agentsPeptide/protein ingredientsNucleotideLyase
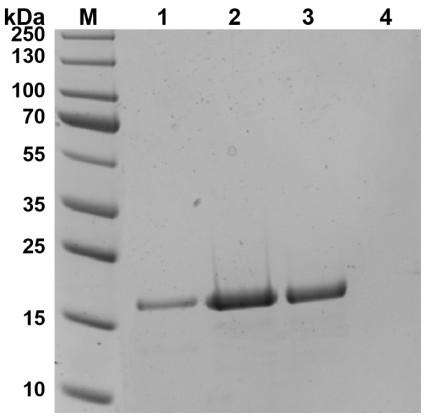
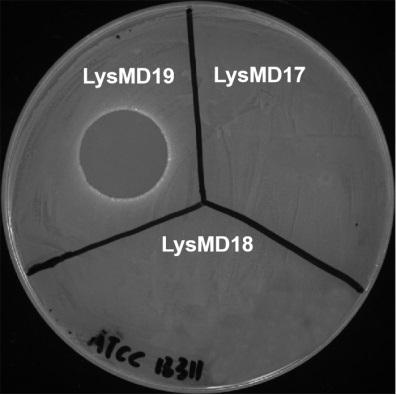
The invention relates to salmonella broad-spectrum lyase with in-vitro lytic activity and application thereof, and belongs to the technical field of biology. The lyase is named as LysMD19, an amino acid sequence of the lyase is shown as SEQ ID No. 1, and a nucleotide sequence of the lyase is shown as SEQ ID No. 2. The lyase can directly and efficiently lyse salmonella in-vitro under the conditionof no cell permeation reagents such as ETDA, the lysing effect is achieved on salmonella enteritidis, salmonella typhimurium, salmonella pullorum and other salmonella of different serotypes, good temperature stability and high acid and alkali resistance are achieved, the lyase can be used for preventing and treating salmonella infection, and also can be used for killing salmonella in multiple links such as source farms, processing, packaging and transportation to market selling, the safety 'from farms to dining tables' is ensured, and good application prospects and values are achieved.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Chicken ring RNAChr26:2670958/2679178 detection primer, method and application
InactiveCN107988404AMicrobiological testing/measurementAgainst vector-borne diseasesNucleotideNucleotide sequencing
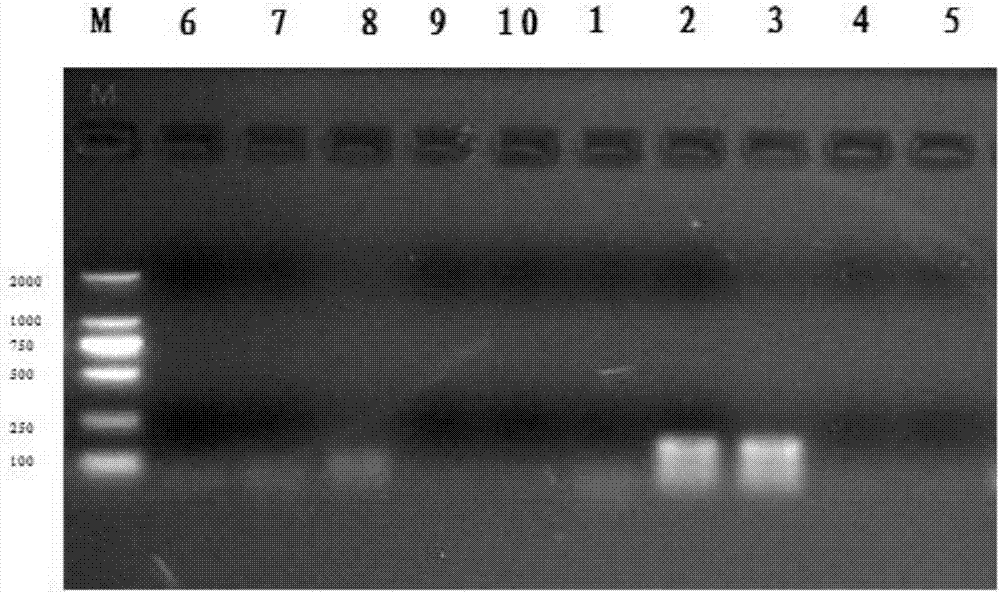
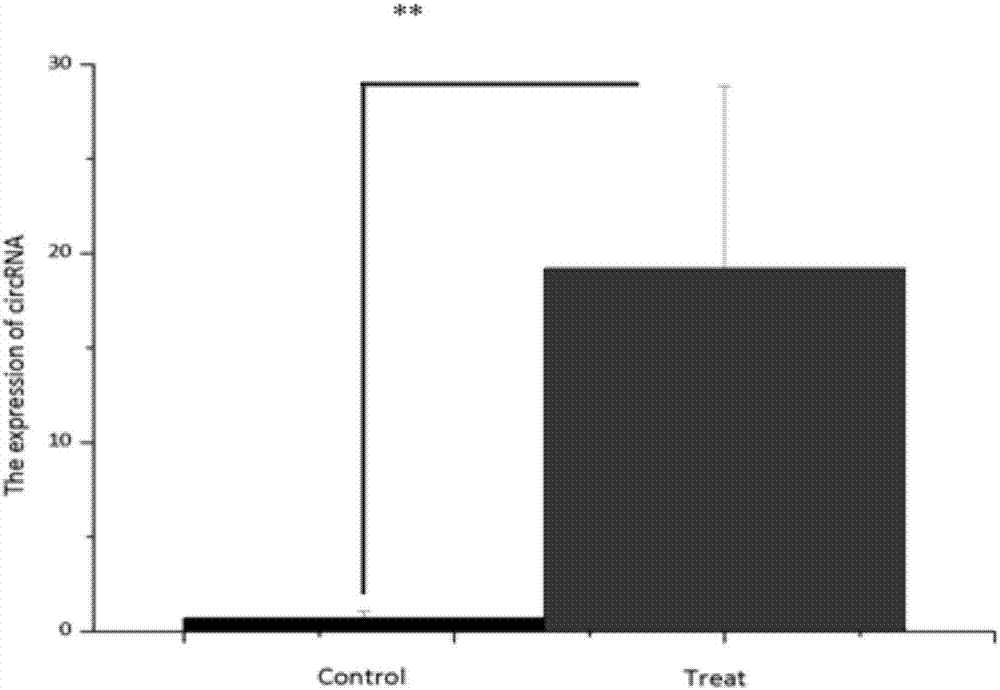
The invention discloses a chicken ring RNA Chr26:2670958 / 2679178 detection primer, a method and application. The primer is prepared from a chicken ring RNA Chr26: 2670958 / 2679178 annular upstream primer body and a chicken ring RNA Chr26: 2670958 / 2679178 annular downstream primer body, wherein nucleotide sequences of the chicken ring RNA Chr26: 2670958 / 2679178 annular upstream primer body and the chicken ring RNA Chr26: 2670958 / 2679178 annular downstream primer body are respectively shown in SEQ ID NO.1 and SEQ ID NO.2. By means of the method disclosed by the invention, the relative expressionsituation of the chicken ring RNA Chr26: 2670958 / 2679178 can be accurately detected after a chicken is infected by salmonella enteritidis; the relative expression situation is utilized as a molecularmarker to identify salmonella infection resistance individuals; thus, theoretical basis and scientific basis are provided for salmonella enteritidis infection resisting genetic breeding of chickens.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
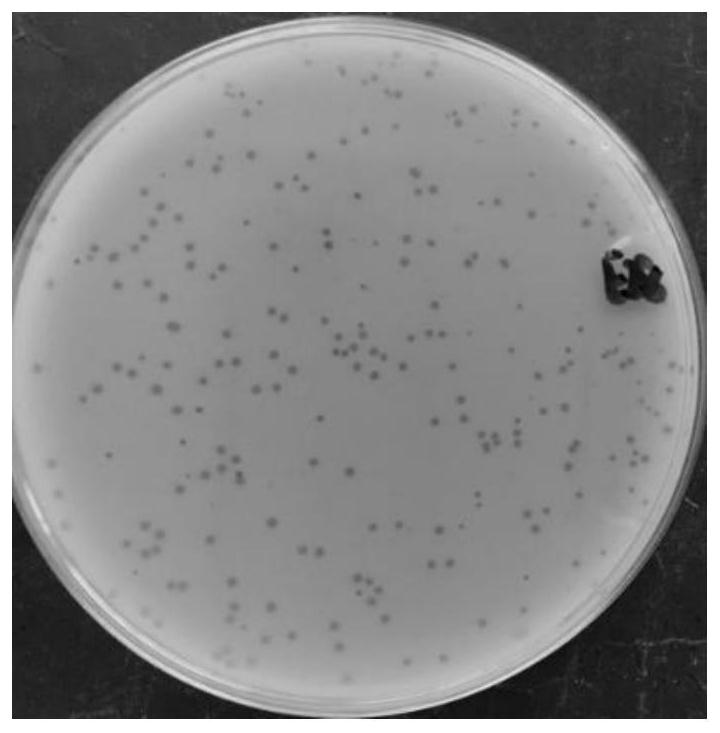
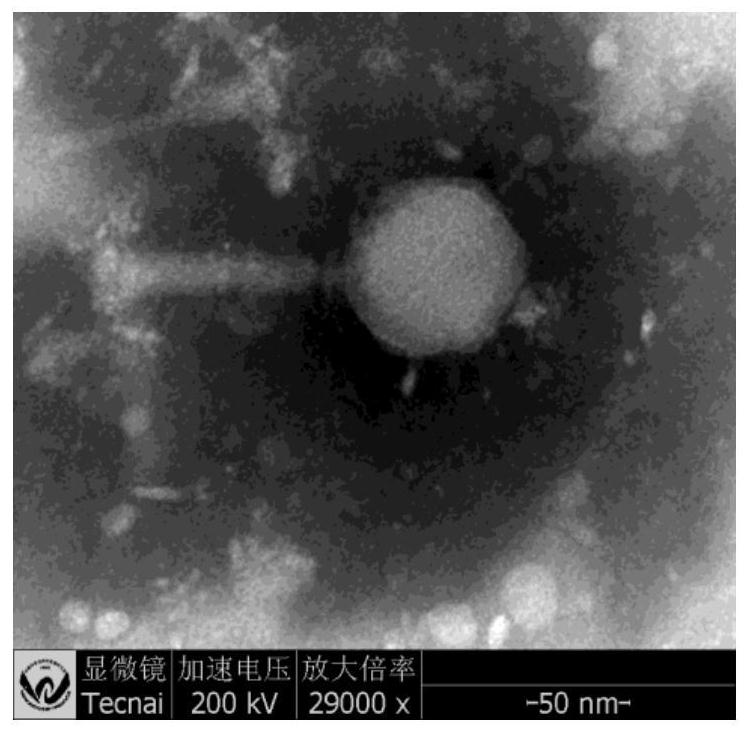
Salmonella phage SEE-1 and application thereof
InactiveCN110904054AGood for universality and effectivenessHost spectrum widthMicroorganism based processesBacteriophagesSalmonella PhageEnteritides
The invention discloses salmonella phage SEE-1 and an application thereof, relates to prevention and control of salmonella, and belongs to the field of bioengineering. A novel bacteriophage SEE-1 is separated from a sewage treatment system, the preservation No. is CGMCC NO.1834D, the basic property of genomes is analyzed, and particularly, functional genes and protein are predicted and analyzed. The separated salmonella phage SEE-1 shows quick and efficient splitting efficiency in experiment of splitting the bacterium enteritidis, and can be used for preventing acterium enteritidis or drug resistant acterium enteritidis in the sewage treatment system.
Owner:UNIVERSITY OF CHINESE ACADEMY OF SCIENCES
Salmonella enteritidis bacteriophage and application thereof
PendingCN112760295AEfficient crackingRich species bankBiocideMicroorganism based processesSalmonella entericaMicroorganism
The invention relates to a salmonella enteritidis bacteriophage and application thereof, and belongs to the technical field of biology. A strong lytic bacteriophage is characterized in that the collection number of the bacteriophage is GDMCC No: 61171-B1, the bacteriophage is collected in Guangdong Microbiological Culture Collection Center on August 25, 2020, the taxonomic name of the bacteriophage is salmonella enteritidis subspecies, namely salmonella enterica subsp.enterica bacteriophage, and the bacteriophage has an efficient bactericidal ability on salmonella enteritidis.
Owner:GUANGDONG MEDICAL UNIV
Composition comprising bacteriophages for reducing, eliminating and/or preventing salmonella enteritidis, salmonella typhimurium and salmonella paratyphi b
ActiveUS20190070231A1Inhibition of colonizationBlock cycle of colonizationAntibacterial agentsPeptide/protein ingredientsSlaughter houseDisinfectant
The present invention relates to a new cocktail of bacteriophages with specific lytic activity against Salmonella enteritidis, Salmonella typhimurium and Salmonella paratyphi B., for reducing, eliminating and / or preventing them in farm animals and animals from the poultry sector, such as poultry, hens and breeding hens, in addition to eggs. It may be administered as an additive in the feed, in water or by spray. Moreover, the cocktail may be used as a disinfectant in work areas of farms and abattoirs, and in processed foods, without affecting the organoleptic properties of the product.
Owner:UNIV DE LOS ANDES
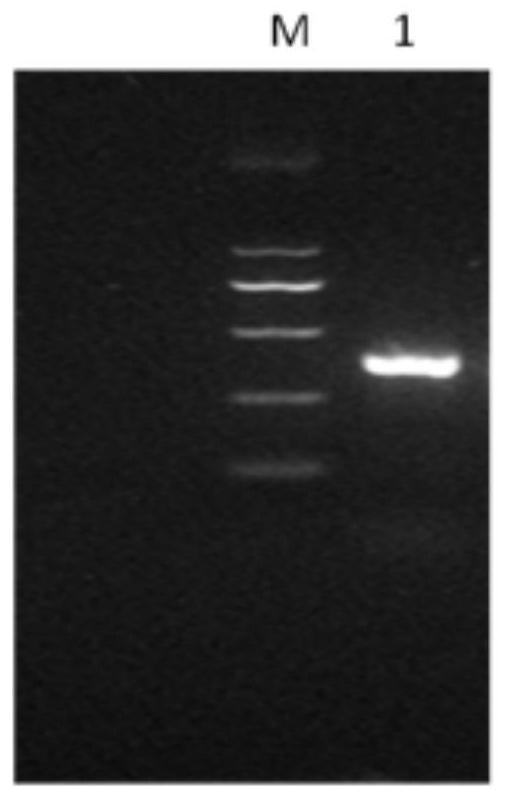
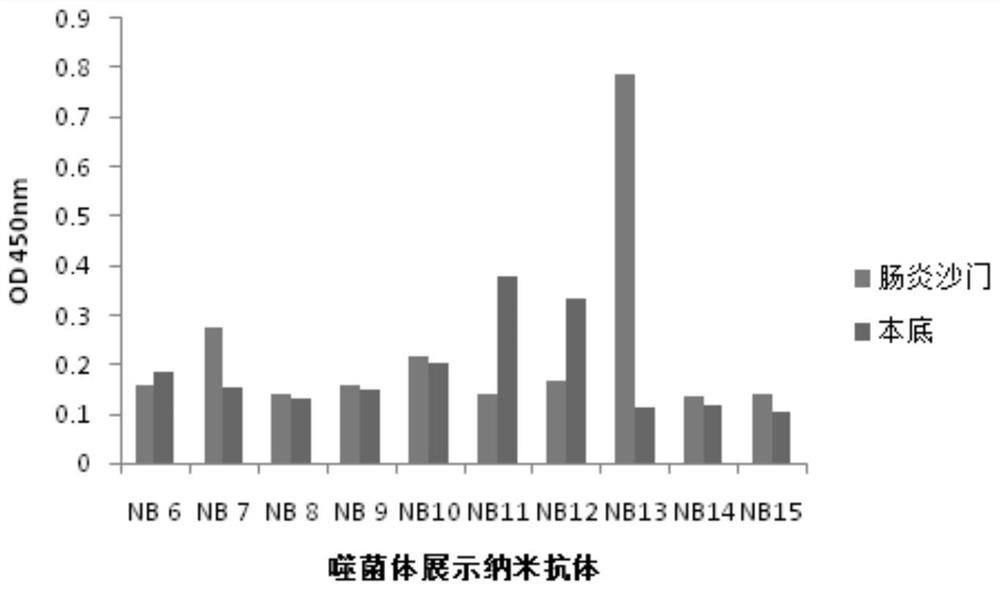
A nanobody against Salmonella enteritidis and its application
ActiveCN108864281BUnique variable domain sequenceSpecific recognition abilityImmunoglobulins against bacteriaFermentationAntiendomysial antibodiesSalmonella diarizonae
The invention relates to a nanometer antibody for resisting salmonella enteritidis and application thereof. The antibody has the function of being combined with the salmonella enteritidis. The invention also discloses the nanometer antibody and a gene sequence for coding the nanometer antibody, an expression carrier and a host cell of the nanometer antibody, and a method for producing the nanometer antibody. The nanometer antibody for resisting the salmonella enteritidis has the advantages that the size is small; the expression efficiency is high; the dissolution performance is good; the stability is high, and the like. The invention also provides the application of the nanometer antibody for resisting the salmonella enteritidis.
Owner:NORTHWEST A & F UNIV
Composition comprising bacteriophages for reducing, eliminating and/or preventing Salmonella enteritidis, Salmonella typhimurium and Salmonella paratyphi B
Owner:UNIV DE LOS ANDES
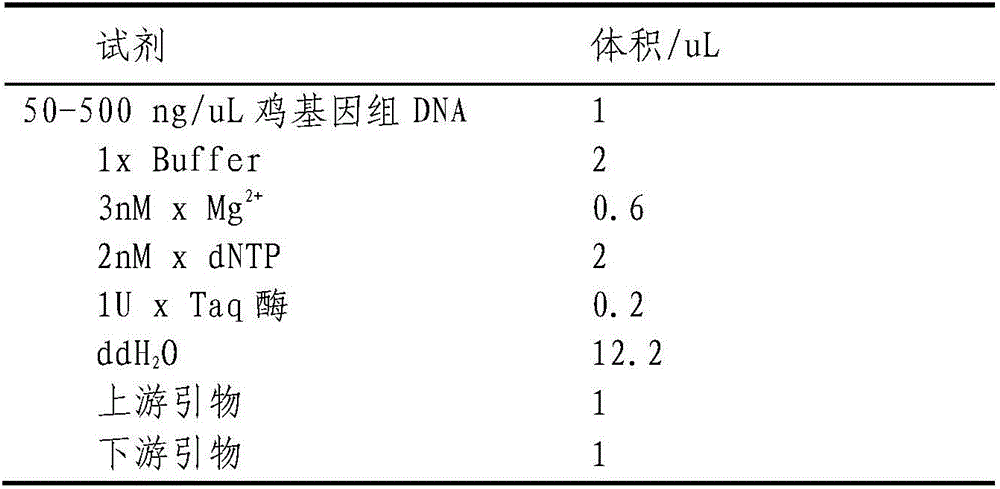
Triple real-time fluorescent PCR detection primers, probes, detection kits and detection methods for three bacteria
ActiveCN102747144BMicrobiological testing/measurementMicroorganism based processesSerotypeEnteritides
The invention discloses a triple real-time fluorescent PCR detection primer, probe, detection kit and detection method for Salmonella, Salmonella enteritidis and Salmonella typhimurium. The present invention utilizes a triple real-time fluorescent PCR method to use the aceA gene of Salmonella to detect Salmonella of different serotypes, the specific sequence of Salmonella enteritidis to detect Salmonella enteritidis, and the STM4599 sequence of Salmonella typhimurium to specifically detect Salmonella typhimurium, and optimize the reaction Conditions, through a real-time fluorescent PCR amplification to determine whether the sample is contaminated by Salmonella, Salmonella enteritidis and Salmonella typhimurium, the detection is fast, and the process from preparing the sample to issuing the test result can be completed within 30 hours, free from false positives and cross-contamination and other interference, the results are reliable, and the sensitivity and specificity are strong, which provides a favorable tool for the epidemiological investigation of Salmonella.
Owner:许龙岩 +5
Recombinant bacterium for expressing SEF14 functional fimbriae and application of recombinant bacterium
ActiveCN112481287AHas agglutinationSpecific and fastBacteriaMicrobiological testing/measurementSalmonella diarizonaeEnteritides
The invention discloses a recombinant plasmid. The recombinant plasmid is obtained by inserting a sef14 operon gene into an expression vector pBR322. The invention also discloses a recombinant strain.The recombinant strain is obtained by introducing the recombinant plasmid into inert carrier bacteria S9. The invention also discloses a preparation method and an application of the recombinant strain. According to the invention, SEF14 is subjected to surface display on the inert carrier bacterium S9 for the first time to express single fimbriae SEF14, so that background non-specific reaction canbe avoided, salmonella enteritidis infection can be specifically detected at the same time, the method has the advantages of rapidness, specificity, sensitivity, simplicity, low cost and the like, and the requirements of on-site and large-scale detection can be met. The SEF14 functional bacterial hair can be used for detecting and monitoring salmonella enteritidis infected chicken flocks after single bacterial hair SEF14 is shown and expressed on the upper surface of an inert carrier, only 5-10 microliters of the detection sample and an isopyknic detection reagent are needed, the salmonella enteritidis infected chicken flocks can be detected and monitored by utilizing a simple glass plate agglutination reaction, and observing of a reaction result by naked eyes on site and accurately judging whether an animal is infected by salmonella enteritidis or not are carried out.
Owner:YANGZHOU UNIV
A Recombinant Attenuated Salmonella Enteritidis
ActiveCN106754535BGood genetic stabilityGood growth and reproduction performanceBacteriaVirus peptidesRecombinant vaccinesVector vaccine
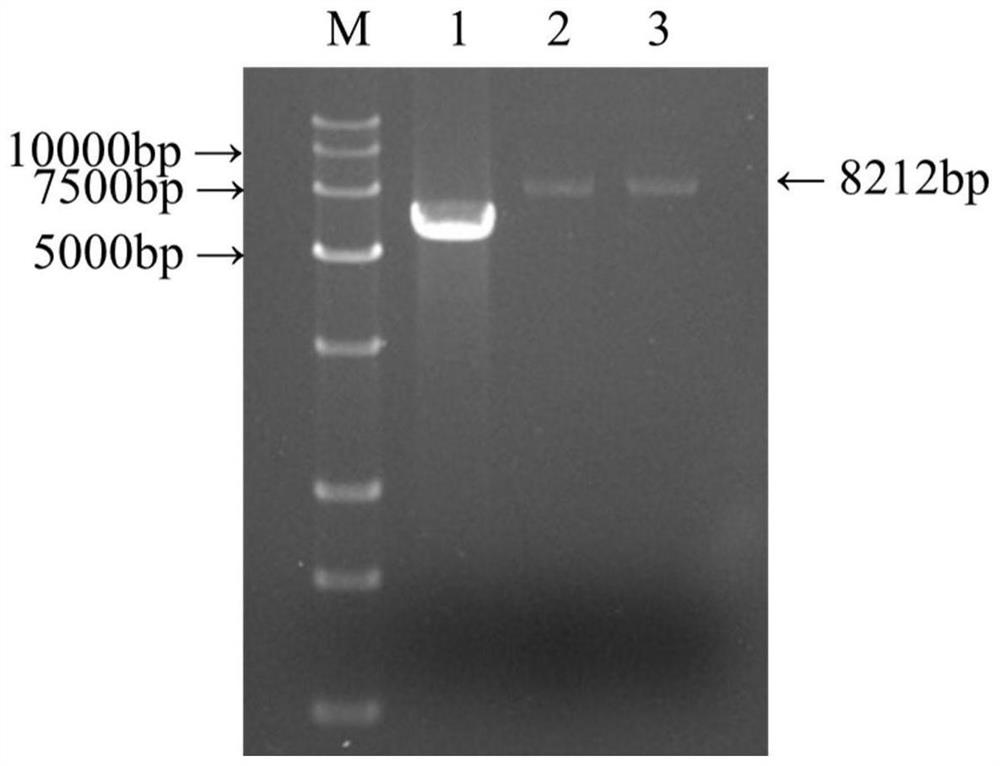
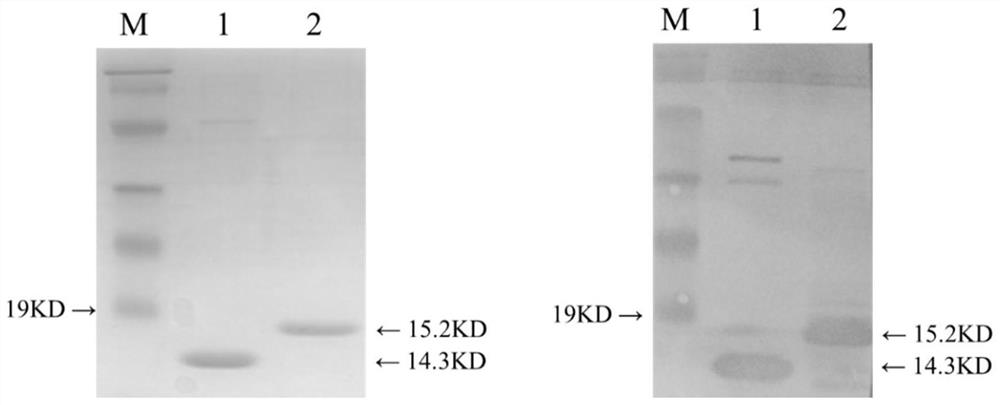
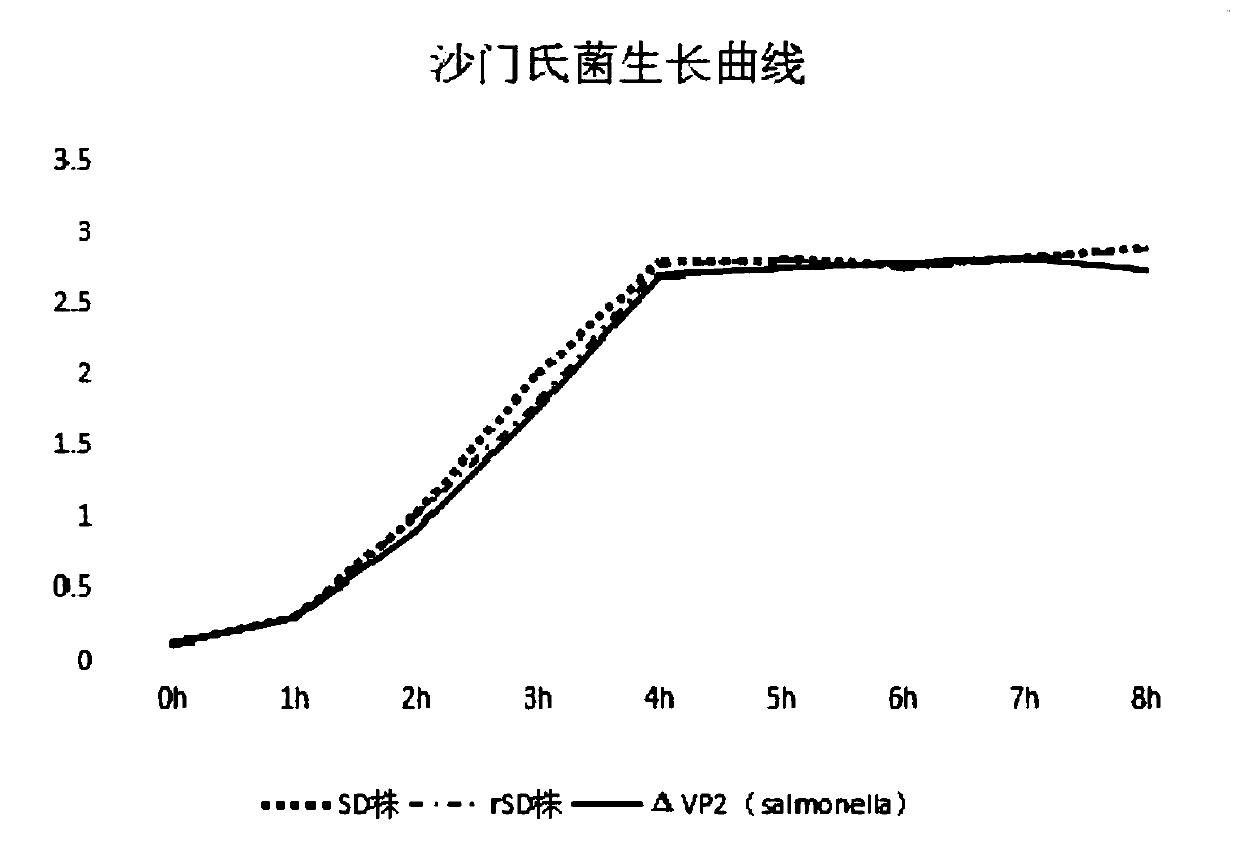
The invention provides a rSD strain of attenuated Salmonella enteritidis, and its preservation number is CGMCC NO.13346. The attenuated Salmonella enteritidis provided by the invention is used to prepare recombinant vaccine strains. Another aspect of the present invention provides a recombinant Salmonella enteritidis live vector vaccine strain, whose genome contains the VP2 gene of IBDV. The preservation number of one of the recombinant Salmonella enteritidis live vector vaccine strains is CGMCC NO.13251. The recombinant vaccine strain constructed by the attenuated Salmonella enteritidis provided by the present invention as a host has good genetic stability. It has been continuously passed on for 20 generations in ordinary medium, and the inserted exogenous gene has not undergone any mutation; the recombinant bacterium does not contain any resistance, which is in line with modern The development trend of bacterial live vaccines has good safety and does not need to consider antibiotic residues. The growth and reproduction performance of the recombinant bacteria is good, and the proliferation of the bacteria is not affected by the insertion of the foreign gene.
Owner:SHANDONG SINDER TECH +1
A strain of Salmonella enteritidis ssrab gene deletion
InactiveCN105018400BDecreased colonization rateReduce the chance of infection with the pathogenBacteriaMicroorganism based processesEnteritidesIn vivo
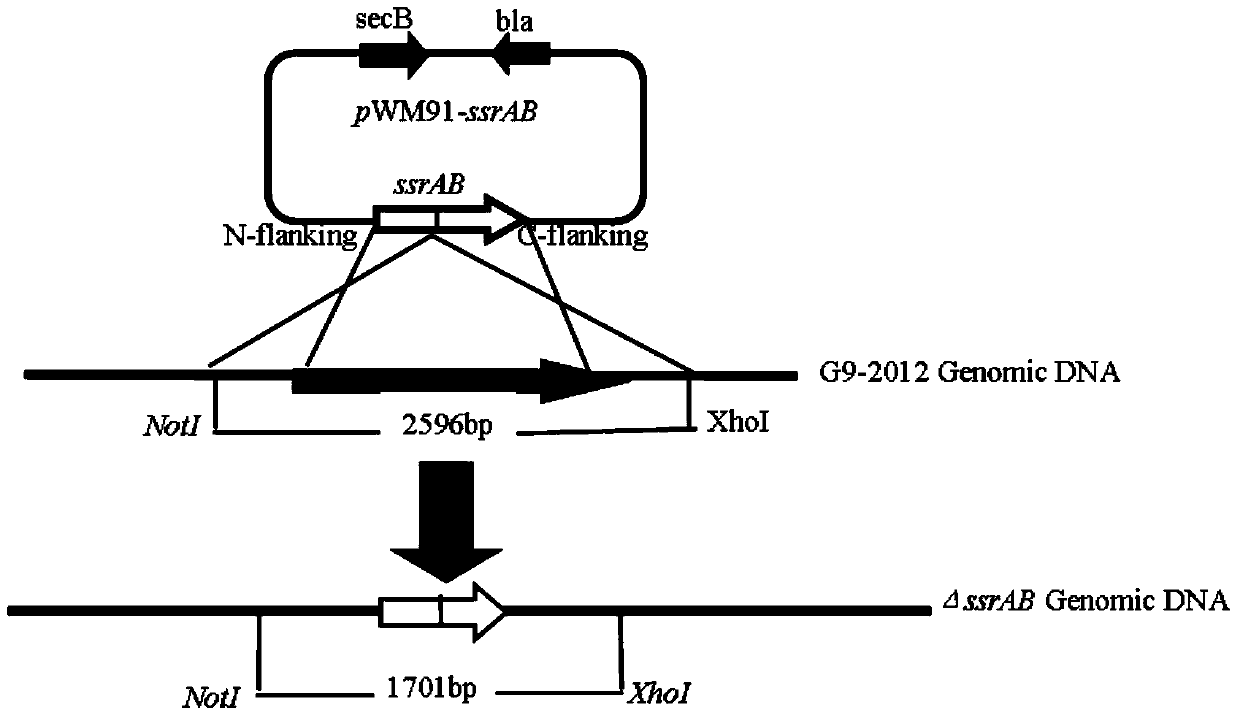
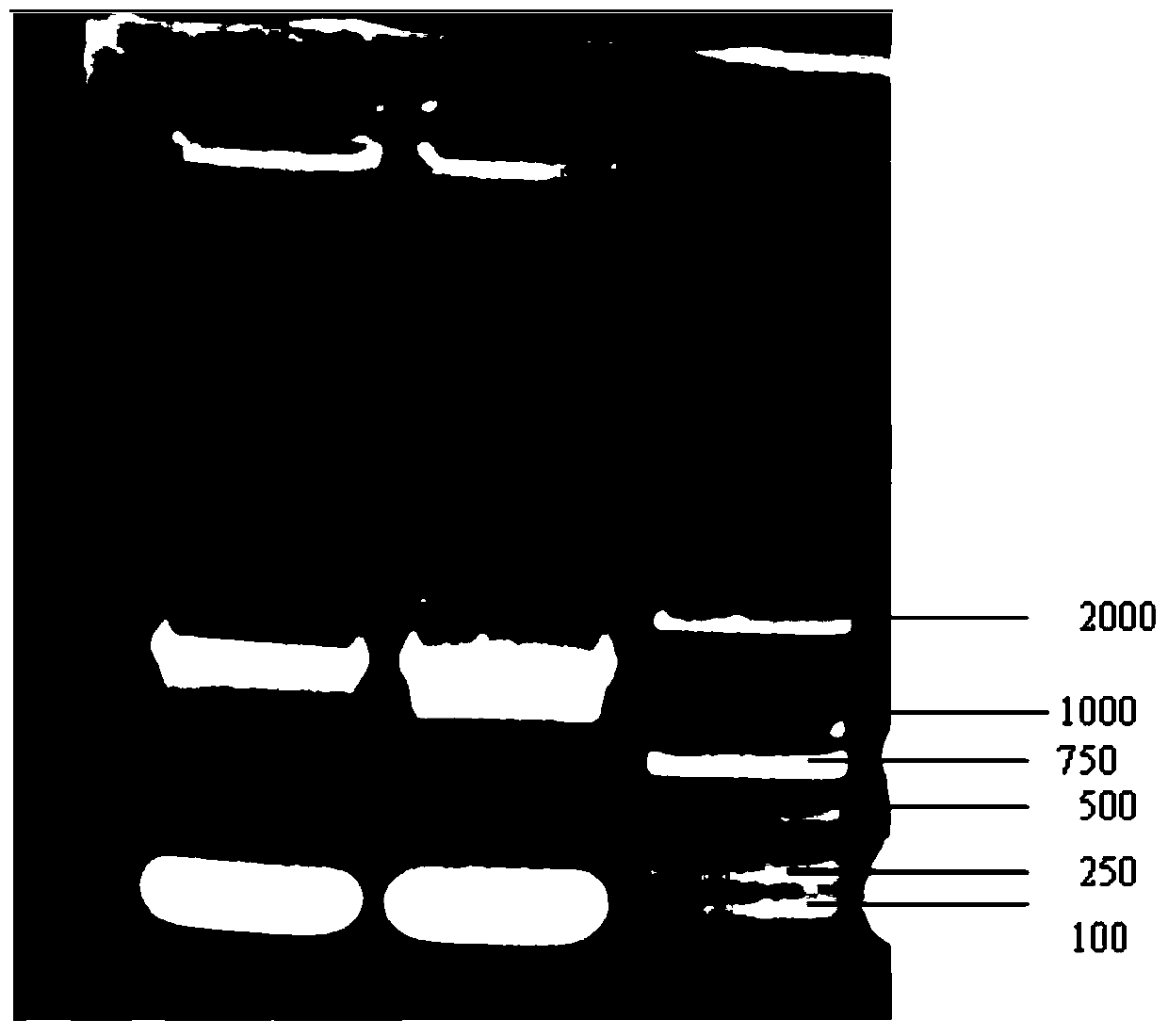
The invention discloses a salmonella enteritidis strain with ssrAB gene deletion. The strain is obtained after knockout of altogether 915 base pairs, from amino acid located at site 689 of ssrA gene to amino acid located at site 63 of ssrB gene. The invention further discloses a construction method for the salmonella enteritidis strain with ssrAB gene deletion. The construction method comprises the following steps: (1) acquiring SN and SC genes of ssrAB and connecting upstream and downstream homologous arms through overlap PCR; (2) constructing recombinant suicide plasmid pWM91-ssrAB; (3) carrying out solid phase joint of bacteria; and (4) screening and acquiring G9-2012 (delta ssrAB). The invention has the following beneficial effects: the G9-2012 (delta ssrAB) strain has a decreased colonization rate and obviously weakened virulence in vivo, can be used for development of attenuated live vaccine or live vector vaccine against salmonella enteritidis, and thus reduces the rate of infection of human beings or animals by salmonella enteritidis and guarantees health of the mankind.
Owner:史记生物技术有限公司
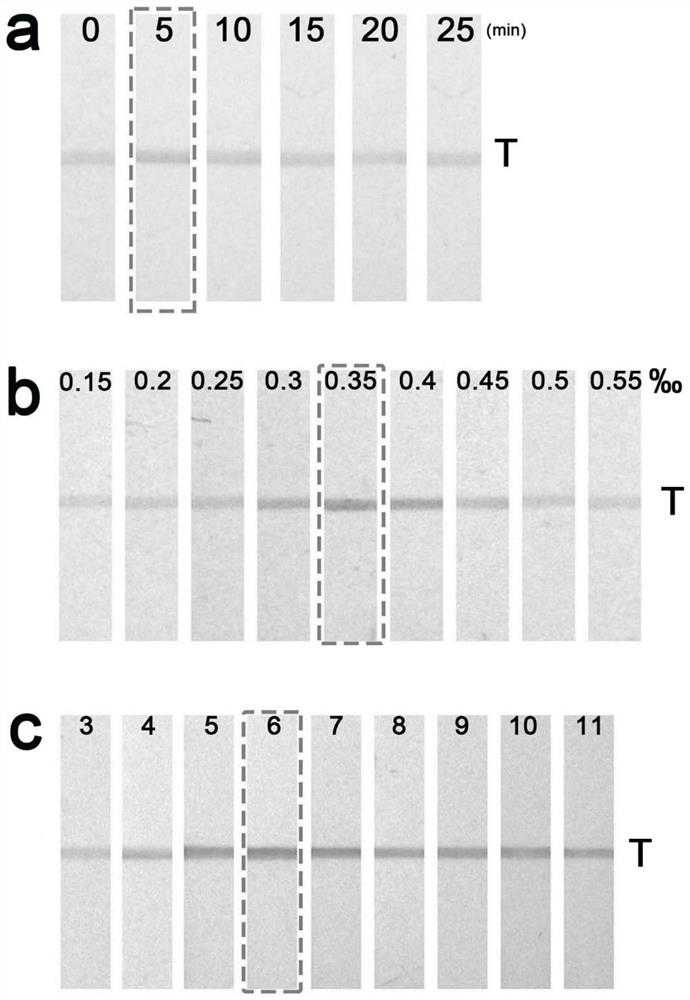
A kind of detection method, test strip and application of Salmonella enteritidis
ActiveCN109633151BEliminate the marking processLow costBiological material analysisCelluloseCarbon Nanoparticles
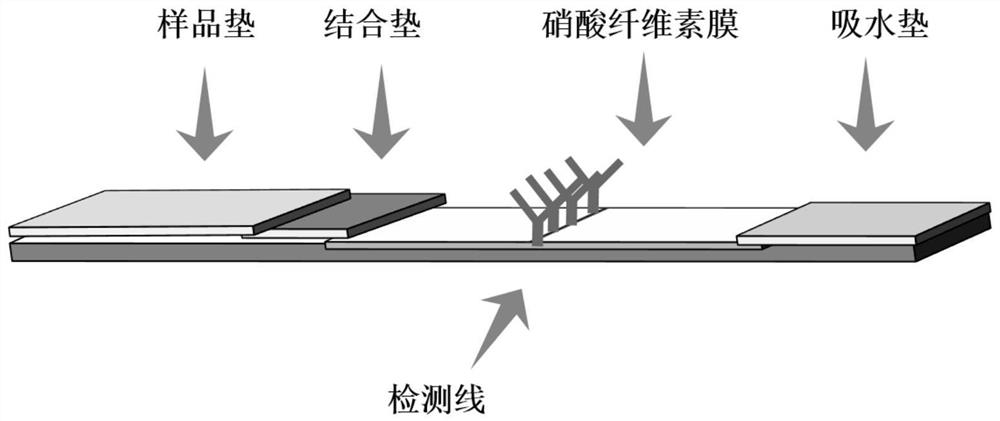
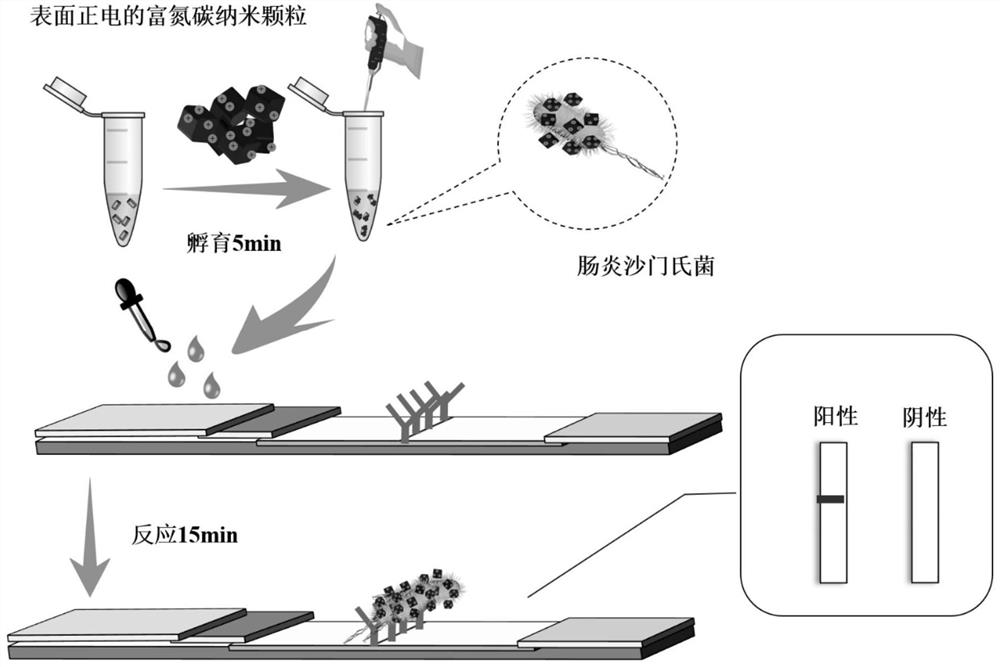
The invention discloses a method for detecting Salmonella enteritidis, a test strip and its application. The method for detecting Salmonella enteritidis comprises incubating positively charged nitrogen-rich carbon nanoparticles with Salmonella enteritidis in a sample to be detected to obtain a solution to be tested, and then The Salmonella enteritidis bound to the positively charged nitrogen-rich carbon nanoparticles in the test solution is captured by the Salmonella enteritidis monoclonal antibody. The invention only uses one antibody to draw on the nitrocellulose membrane for direct detection, which breaks the traditional sandwich detection method that uses two antibodies at the same time, greatly saves the cost, solves the difficulty of pairing antibodies, and is simpler, more convenient and novel.
Owner:NORTHWEST A & F UNIV
A Salmonella enteritidis phage lpse28 and its application in food
ActiveCN108546685BStock solution titer is highStrong bacteriostasisMilk preservationMicroorganism based processesBiotechnologySalmonella Phage
The invention discloses a salmonella enteritidis phage LPSE28, with the preservation number of CCTCC NO. 2018120. The salmonella enteritidis phage adopts a wide-spectrum type and can perform pyrolysison a drug-resistant strain of salmonella. Through verification, the salmonella enteritidis phage belongs to caudovirales myoviridaeT4-like viruses, and is called after LPSE28. The pH value of the phage LPSE28 is between 4 and 12, and the titer of the phage is stable at 40-60 DEG C. The invention also discloses application of the salmonella enteritidis phage LPSE28 to food. By utilization of the phage provided by the invention, salmonella enteritidis in food, particularly liquid eggs and milk systems can be effectively controlled, and compared with an antibiotic and a chemical preservative, the phage LPSE28 has the characteristics of high specificity, no residues and safety.
Owner:HUAZHONG AGRI UNIV
Detection method and application of chicken Salmonella enteritidis infection resistance molecular marker
ActiveCN106636331AAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesGenotypeLRP5
The invention relates to the technical field of gene engineering, and provides a detection method and application of a chicken Salmonella enteritidis infection resistance molecular marker-related gene LRP5. The method provides a chicken LRP5 gene rs80757564 site as the molecular marker, and correspondingly provides typing primers and corresponding PCR (polymerase chain reaction) conditions. The primers and method can be utilized to accurately detect the genotype of the LRP5 gene after the chickens are infected by Salmonella enteritidis. The individual with high Salmonella enteritidis infection resistance is selected to provide theoretical basis for chicken Salmonella-enteritidis-resistant breeding.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Dual fluorescent PCR primer group, kit and method for detecting salmonella enteritidis and mycoplasma synoviae
PendingCN114574605ANo non-specific bindingEasy to operateMicrobiological testing/measurementMicroorganism based processesMycoplasma synoviaeSalmonella diarizonae
The invention discloses a dual fluorescence PCR primer group, a kit and a method for detecting salmonella enteritidis and mycoplasma synoviae, the primer group provided by the invention comprises specific primers aiming at salmonella enteritidis and mycoplasma synoviae, the length of each primer is about 20 basic groups, the GC content is 40-60%, no complementary sequence exists among the primers, and the specific primers can be used for detecting the salmonella enteritidis and mycoplasma synoviae in the salmonella enteritidis and mycoplasma synoviae in the salmonella enteritidis and mycoplasma synoviae in the salmonella enteritidis and mycoplasma synoviae. The melting temperature difference is less than 5 DEG C, and no complementary sequence or secondary structure exists in the primer. The kit provided by the invention simultaneously contains the primer groups of the salmonella enteritidis and the mycoplasma synoviae, the four primers do not interfere with one another during amplification, two pathogens can be screened by one-time amplification reaction by using the kit provided by the invention, the defect that an existing kit cannot simultaneously detect the salmonella enteritidis and the mycoplasma synoviae is effectively overcome, and the kit is suitable for large-scale popularization and application of the salmonella enteritidis and the mycoplasma synoviae. Powerful technical support is provided for purification of chicken enteritis salmonellosis and mycoplasma synoviae in chicken farms, and the method has high practical value and wide application prospect.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Application of pyroptosis-related protein gsdmd in preparation of slough vaccine
ActiveCN109303916BAvoid damageInfection controlAntibacterial agentsPeptide/protein ingredientsEnteritidesHumoral immune reaction
The invention discloses the application of the pyroptosis related protein GSDMD in the preparation of the slough vaccine. The present invention constructs the lysogenic plasmid containing the coding gene of pyroptosis-related protein GSDMD, transfers it into Salmonella enteritidis, lyses the bacteria under the induction of arabinose, and successfully obtains the novel slough vaccine of Salmonella. Experiments have proved that the bacteriolysis mediated by GSDMD has an extremely long duration, and the lysis rate reaches more than 99.9985%. After immunizing mice with the novel slough prepared by the present invention, it can stimulate the body to produce strong humoral immunity and cellular immunity, induce a protective immune response and protect experimental animals against Salmonella infection, indicating that the Salmonella slough prepared by the present invention The vaccine has a good immune protection effect.
Owner:HARBIN WEIKE BIOTECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com