Avian adenovirus, quadruple vaccine and preparation method thereof
An avian adenovirus, avian influenza virus technology, applied in the direction of biochemical equipment and methods, viruses, vaccines, etc., can solve the problems of economic loss, harm, severe immunosuppression, etc., achieve small immune dose, rapid antibody production, immunogenicity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
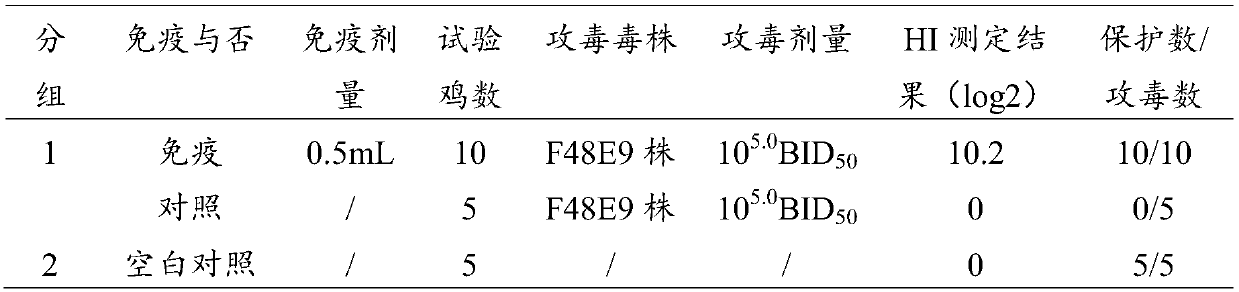
[0028] Example 1 Screening of Antigen Strain (I Avian Adenovirus Group I Serum 4DC Strain) Used for Seedling Production
[0029] In 2015, liver disease material was taken from a dead chicken in a chicken farm in Dachang, Hebei (necropsy showed hepatomegaly, necrosis, and a large amount of yellow fluid in the pericardium), and was inoculated with the yolk sac route for 6 days after grinding, homogenizing, centrifuging, and filtering Age SPF chicken embryos were used for virus isolation. Chicken embryos were all dead 96 hours after inoculation with the disease material, and the embryo body of the chicken embryos had dysplasia, congestion and hemorrhage, liver mottled necrosis and other lesions. The dead embryos were harvested and homogenized with saline at 1:3 to make a virus suspension. The virus suspension continued to be passed on chicken embryos for 2 times, and then inoculated with LMH cells for 3 generations to obtain an isolated virus strain adapted to the growth of LMH c...
Embodiment 2
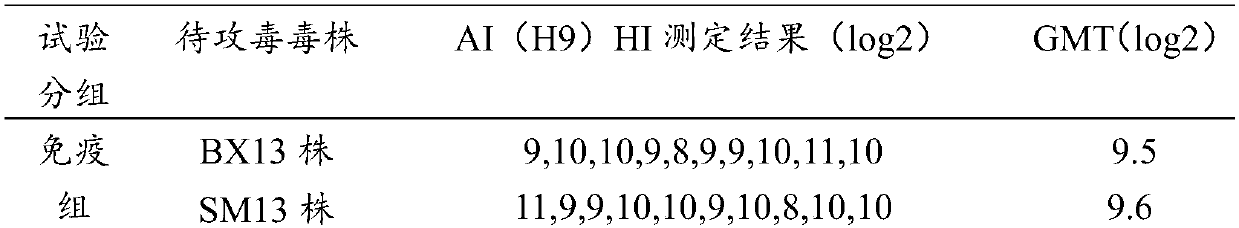
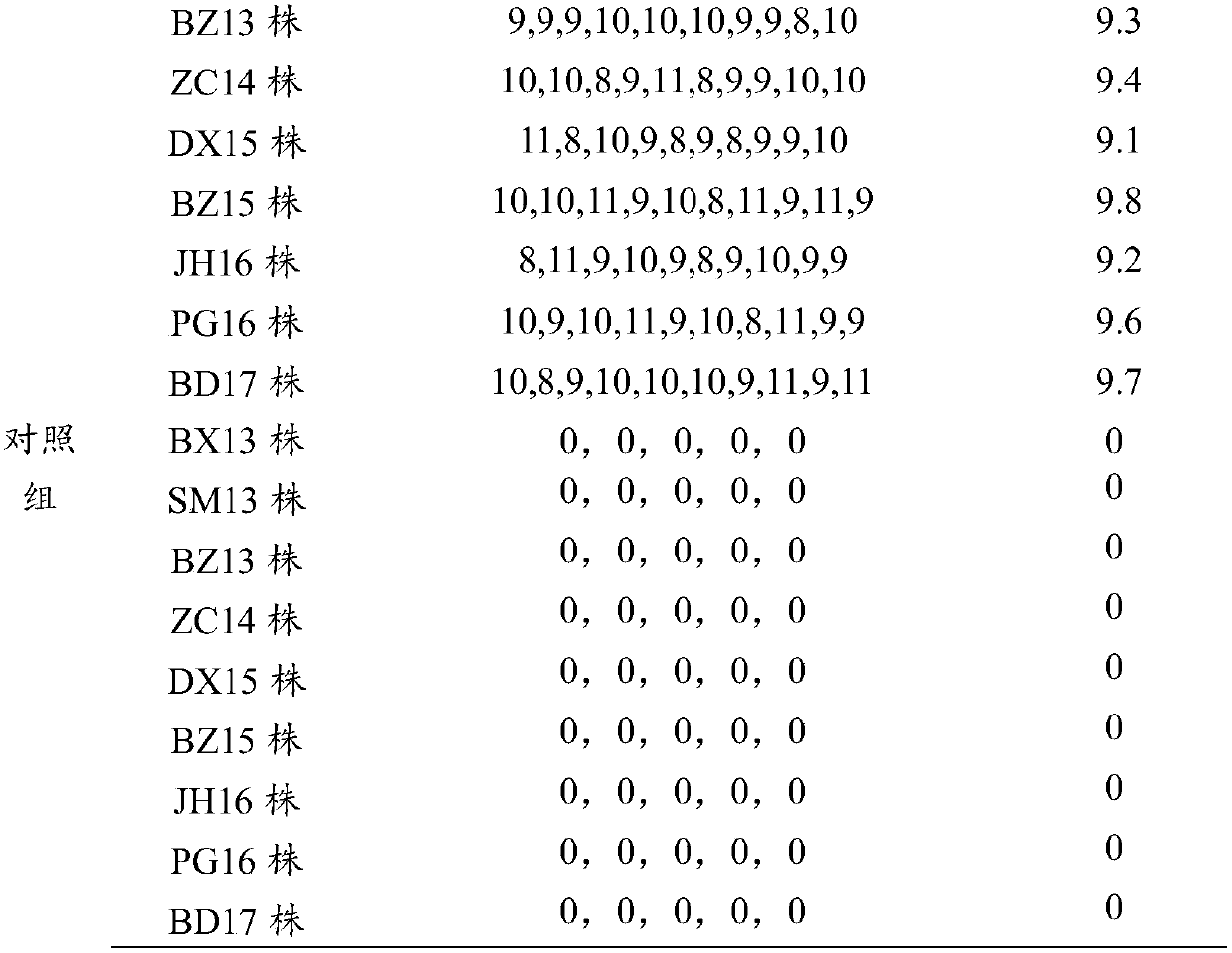
[0032] Embodiment 2 Preparation and inspection of chicken Newcastle disease, avian influenza, infectious bursal and avian adenovirus quadruple vaccine
[0033] 1. Preparation of Antigen Solution for Seedling Production
[0034] 1.1 Preparation of avian influenza (H9 subtype) antigen solution
[0035] 1.1.1 Avian influenza (H9 subtype) BX13 strain was isolated and preserved by the Animal Husbandry and Veterinary Research Institute of Beijing Academy of Agriculture and Forestry Sciences in 2013 from a sick chicken flock with H9 subtype avian influenza in Beijing. After systematic identification, it was proved to be H9N2 subtype type of avian influenza virus. The propagation of virus gets the poison seed for production, and makes appropriate dilution (such as 10 -3 to 10 -5diluted), inoculate 0.1 mL into the allantoic cavity of each embryo, seal the pinhole, and continue to incubate at 36-37°C without turning the eggs. Incubation and observation Within 48 hours after inoculat...
Embodiment 3
[0087] 1. Preparation of inactivated vaccine with oil adjuvant
[0088] 1. Oil phase preparation
[0089] Take 94 parts by weight of white oil for injection, add 2% (weight ratio) of aluminum stearate, heat, stir while adding, until completely transparent, then add 6 parts by weight of Siben-80, after mixing, autoclave spare.
[0090] 2. Aqueous phase preparation
[0091] Mix the concentrated and inactivated NDV, AIV, IBDV and FADV virus liquid in an appropriate ratio (1:1:3:1), so that the NDV antigen content in each final product is ≥10 8.2 EID 50 、AIV antigen content ≥10 8.2 EID 50 、IBDV antigen content ≥10 8.2 TCID 50 、FADV antigen content ≥10 7.2 TCID 50 Take 96 parts by weight of the mixed antigen solution, add 4 parts by weight of sterilized Tween-80, and shake well until the Tween-80 is completely dissolved.
[0092] 3. Emulsification
[0093] Pour 2 parts by weight of the oil phase into the emulsification tank, stir slowly while slowly adding 1 part of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com