Avian adenovirus transfer carrier and preparation method thereof
A technology for transferring vectors and avian adenoviruses, applied in the field of genetic engineering, can solve problems such as low recombination rate, and achieve the effect of wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1I
[0066] Example 1 Type I avian adenovirus AV208 strain
[0067] 1 Materials and methods
[0068] 1.1 Materials
[0069] Type Ⅰ avian adenovirus AV208 strain was purchased from China Veterinary Drug Administration, and its TCID50 on chicken embryonic kidney cells (CEK) was 105.5 / 0.5mL. Chicken liver cancer cell line (LMH) was obtained from the Key Laboratory of Medical Molecular Virology, Ministry of Education, Fudan University. DMEM / F12 culture medium, fetal bovine serum, antibiotics, and trypsin were all purchased from GIBCO Company. Low melting point agarose was purchased from PROMEGA.
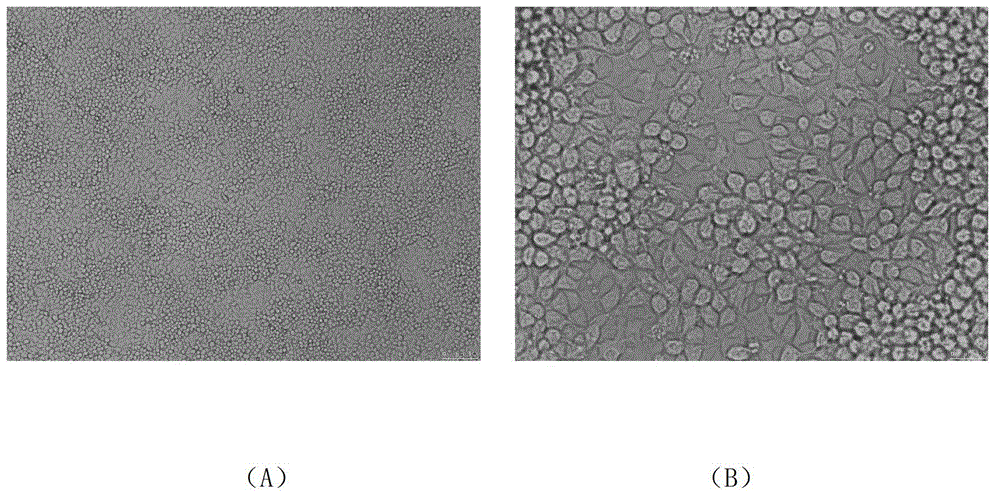
[0070] 1.2 Culture and morphological observation of LMH cells
[0071] LMH cells were cultured in DMEM / F12 medium containing 10% fetal bovine serum and 100 U / mL penicillin and streptomycin, and the cell morphology was observed. In order to explore the culture conditions of this cell line, LMH cells were subcultured at the ratio of 1:3, 1:4, 1:5, and 1:6, and cultured with serum concentra...
Embodiment 2
[0098] Example 2 Construction of Transfer Vector pUC-LR-eGFP
[0099] 1 Materials and methods
[0100] 1.1 Materials
[0101] Type Ⅰ avian adenovirus AV208 strain was purchased from China Veterinary Drug Control Institute. Escherichia coli competent cells DH5α, vectors pMD18-T and pUC18 were purchased from Dalian Bao Biological Engineering Co., Ltd. The pEGFP-N1 plasmid was purchased from Clontech Company. Taq enzyme, T4 DNA ligase, and restriction endonuclease were all purchased from Dalian Bao Biological Engineering Co., Ltd., and Pfu enzyme was purchased from PROMEGA Company. Viral genome extraction kit was purchased from TIANGEN Company. DNA gel recovery kit and plasmid mini-extraction kit were purchased from OMEGA Company.
[0102] 1.2 Extraction of avian adenovirus genome
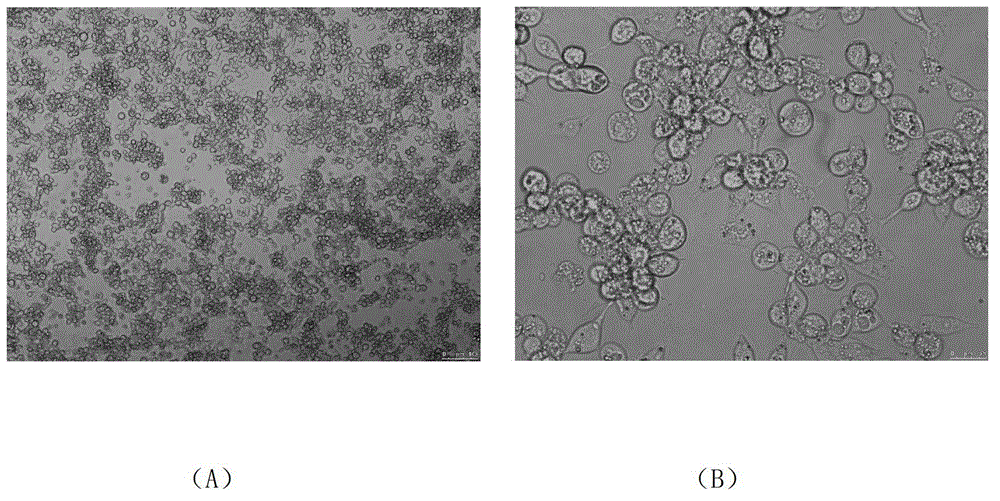
[0103] Avian adenoviruses were obtained from LMH cells infected with FAV-I AV208. After LMH cells have obvious CPE, digest the cells and blow them off with 1ml of medium, harvest them, freeze a...
Embodiment 3
[0226] Construction and screening of embodiment 3 recombinant virus rFAV-I-eGFP
[0227] 1 Materials and methods
[0228] 1.1 Type I avian adenovirus AV208 strain, purchased from China Veterinary Drug Administration, TCID on chicken embryonic kidney cells (CEK) 50 for 10 5.5 / 0.5mL. Chicken liver cancer cell line (LMH) was obtained from the Key Laboratory of Medical Molecular Virology, Ministry of Education, Fudan University. DMEM / F12 culture medium, fetal bovine serum, antibiotics, and trypsin were all purchased from GIBCO Company. Low melting point agarose was purchased from PROMEGA. The endotoxin-free plasmid mini-extraction kit was purchased from OMEGA Corporation. Lipfectamine 2000, a liposome transfection reagent, was purchased from INVITROGEN.
[0229] 1.2 Massive extraction of transfection-grade plasmid pUC-LR-eGFP Take 30ul of pUC-LR-eGFP transformed Escherichia coli DH5αglycerobacteria stored at -20℃ and inoculate 3ml of fresh ampicillin-containing LB culture m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com