Il-33 antagonist-containing therapeutic agent for endometriosis
A technology for endometriosis, applied in medical preparations containing active ingredients, medical preparations with non-active ingredients, anti-animal/human immunoglobulin, etc., can solve uninvestigated endometriosis To achieve the effects of inhibiting fibrosis or proliferation, relieving pain, and inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
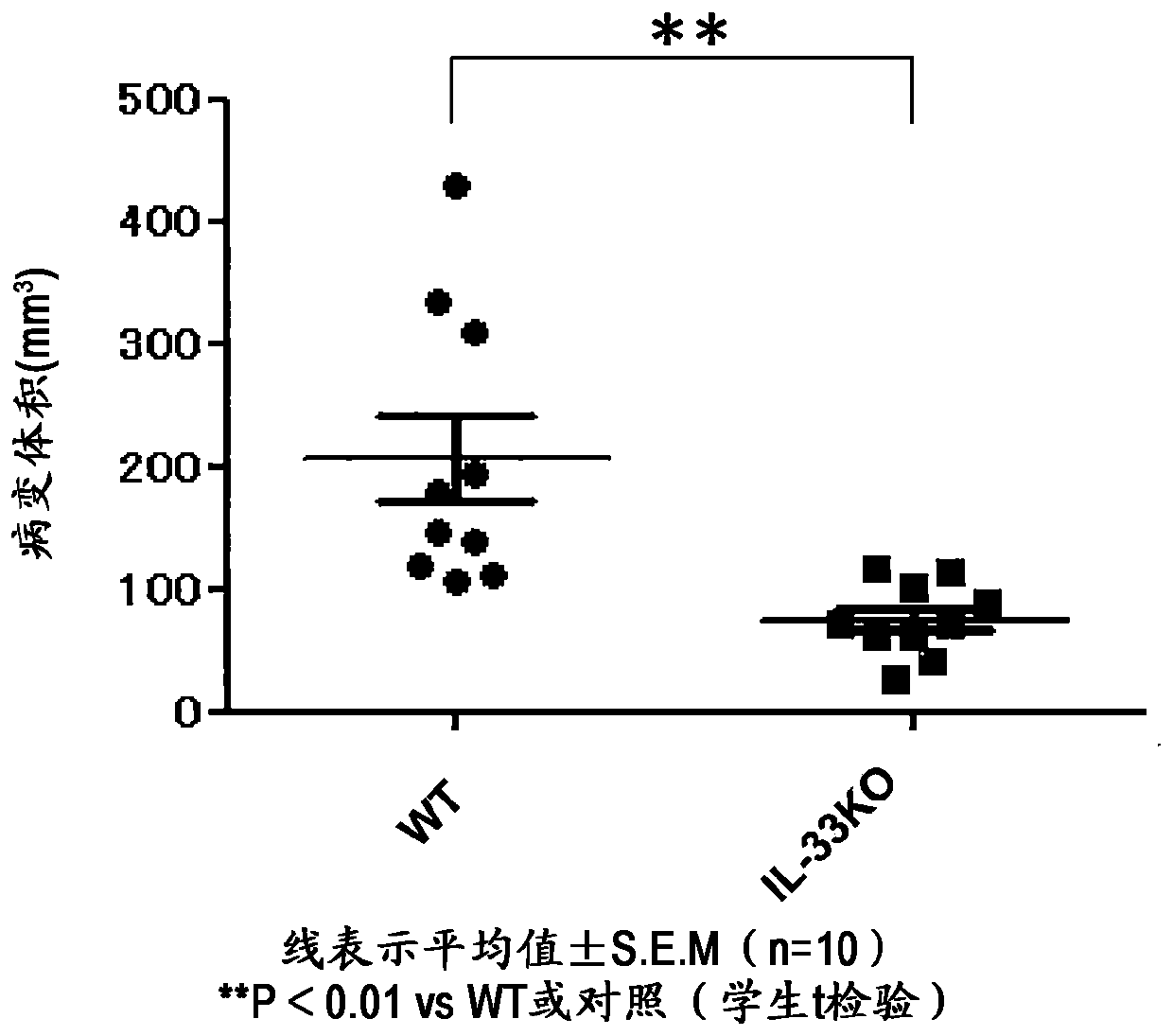
[0142] Example 1: Effect of IL-33 gene defect in endometriosis model
[0143] As an animal model of endometriosis, uterus-transplanted mice administered estrogen were used (Ricci et al., Reprod. Sci. 2011, vol. 18, p614). Using isoflurane (concentration of isoflurane: 3.0%, air flow: 300-400 to maintain anesthesia) on wild-type 6-week-old female Balb / c mice (Charles River, Japan) or Balb / c background IL- 33 knockout mice (Yasuda et al., PNAS, 2012, Vol.109, p3451; Haenuki et al., J. Allergy Clin. Immunol., 2012, Vol.130, p184) (hereinafter referred to as "IL-33KO mice") were performed After inhalation anesthesia was introduced, a small incision was made on the left side of the middle of the lower abdomen of the mouse, and the left and right uteri were pulled out sequentially from the laparotomy hole, and the ovaries attached to the front end of the uterus were removed. For these mice, 0.5 μg / 100 μL / body corn oil for subcutaneous injection (Wako Junyaku Kogyo) was subcutaneo...
Embodiment 2
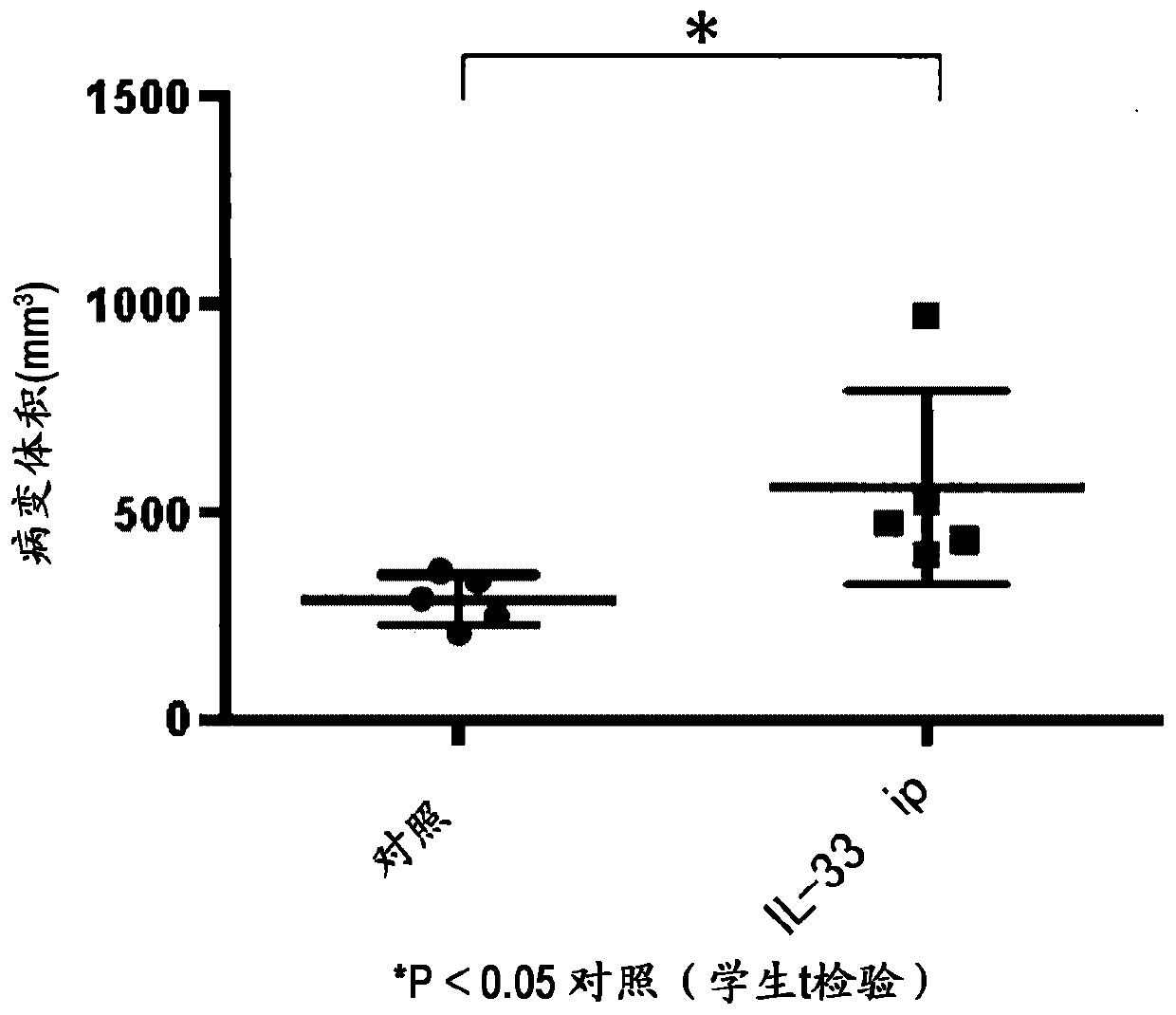
[0145] Example 2: Effect of administration of IL-33 on endometriosis model
[0146] A wild-type 6-week-old female Balb / c mouse was used to prepare an endometriosis model by the method described in Example 1. In the IL-33 administration group, recombinant human IL-33 dissolved in PBS was intraperitoneally administered 1 time at 100 ng / 200 μL / body, 3 times / week, 2 weeks, and 6 times in total from the time of implantation of the uterus protein (residue 112-residue 270) (Kondo et al., Int. Immunol. 2008, vol. 20, p791). Two weeks after the transplantation, the mice were euthanized, the abdomen was opened, and the cyst lesions formed in the abdominal cavity were excised, and the respective volumes were measured. like figure 2 As shown, the volume of the cyst lesion was significantly increased in the human IL-33-administered group compared with the vehicle-administered group. This result shows that IL-33 is involved in the growth of cystic lesions. In addition, the concentrat...
Embodiment 3
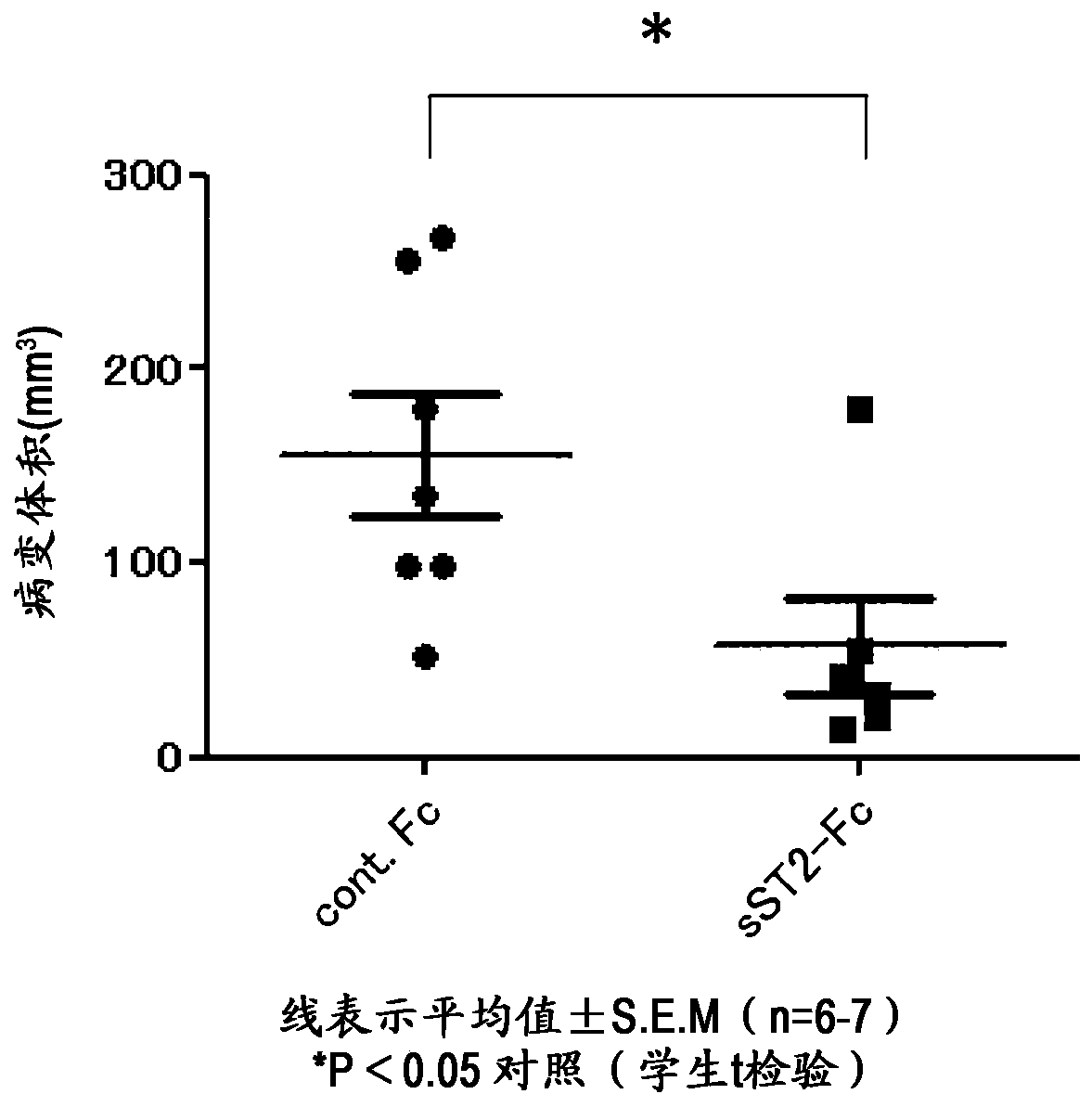
[0147] Example 3: IL-33 antagonists
[0148] Five kinds of human anti-IL-33 antibodies (A10-1C04, A23-1A05, A25-2C02, A25-3H04, A26-1F02) and fusion proteins formed by mouse ST2 and human IgG1 constant region were prepared using recombinant CHO cells. Mouse sST2-Fc (International Publication No. 2015 / 099175). Their amino acid sequences are shown in the table below. The affinity of the five human anti-IL-33 antibodies to human IL-33 protein (residue 112-residue 270) (ATGen, ILC0701) was determined by KinExA. As a result, A10 Kd=100.3pM in -1C04, Kd=195.3pM in A23-1A05, Kd=700fM in A25-2C02, Kd=7.7pM in A25-3H04, Kd=5.3pM in A26-1F02.
[0149] [Table 1]
[0150]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com