Anti-tumor composition
A composition and anti-tumor technology, applied in the direction of anti-tumor drugs, drug combinations, medical preparations containing active ingredients, etc., can solve the problems of complex pathogenic mechanisms and large individual differences among patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
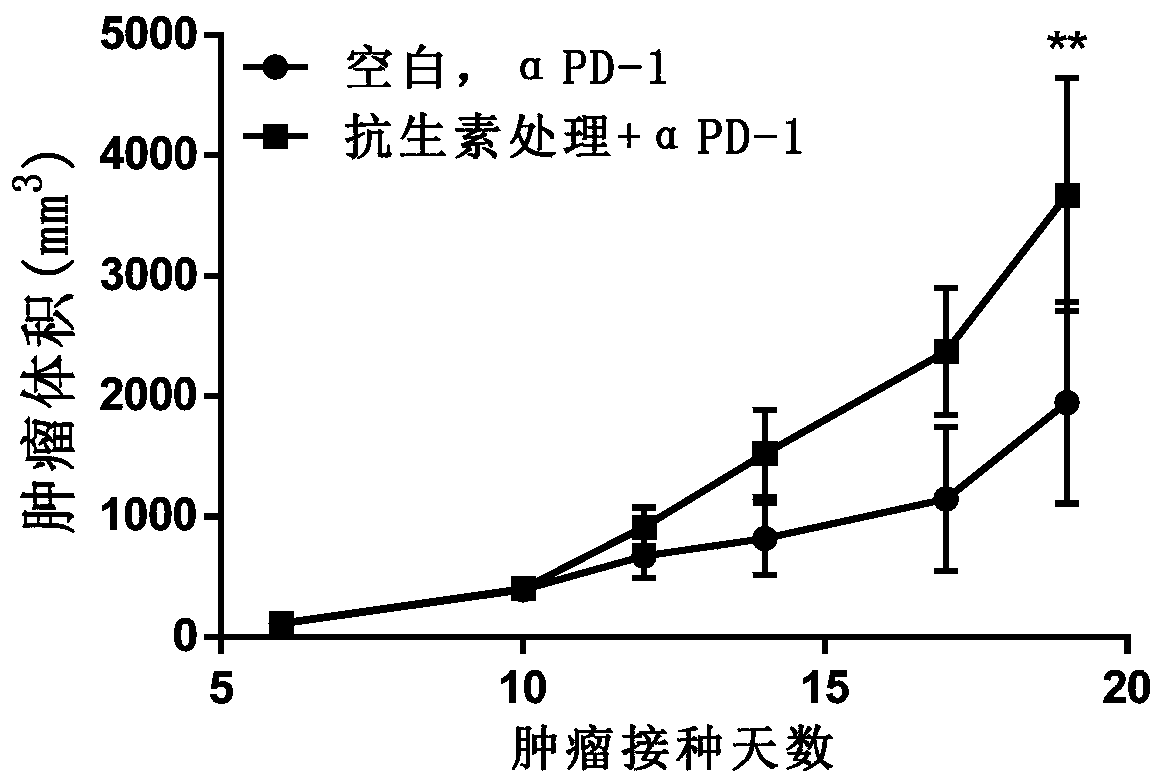
[0072] 24 C57BL / 6J mice at 6-8 weeks were randomly divided into 2 groups according to body weight after one week of acclimatization, with 12 mice in each group, respectively: Group 1 (no antibiotic treatment, anti-mPD-1, 10mg / kg, i.p.), group 2 (antibiotic treatment, anti-mPD-1, 10 mg / kg, i.p.). The antibiotic treatment is oral administration of broad-spectrum antibiotic Ampicillin (1 mg / mL)+colistin (1 mg / mL)+streptomycin (5 mg / mL) for 5 days. Mice in all groups were inoculated with MC38 tumor cells resuspended in phosphate buffered saline (PBS) at a concentration of 1×10 7 per mL, inoculated subcutaneously on the right flank of the experimental animals, and the inoculation dose was 100 μL per animal. Anti-mPD-1 was injected on the 4th day after tumor cell inoculation, once every 4 days, for a total of 4 injections.
[0073] MC38 tumor cells were purchased from Ruting Biotechnology Co., Ltd., with DMEM medium containing inactivated 10% fetal bovine serum, 100 U / mL penicilli...
Embodiment 2
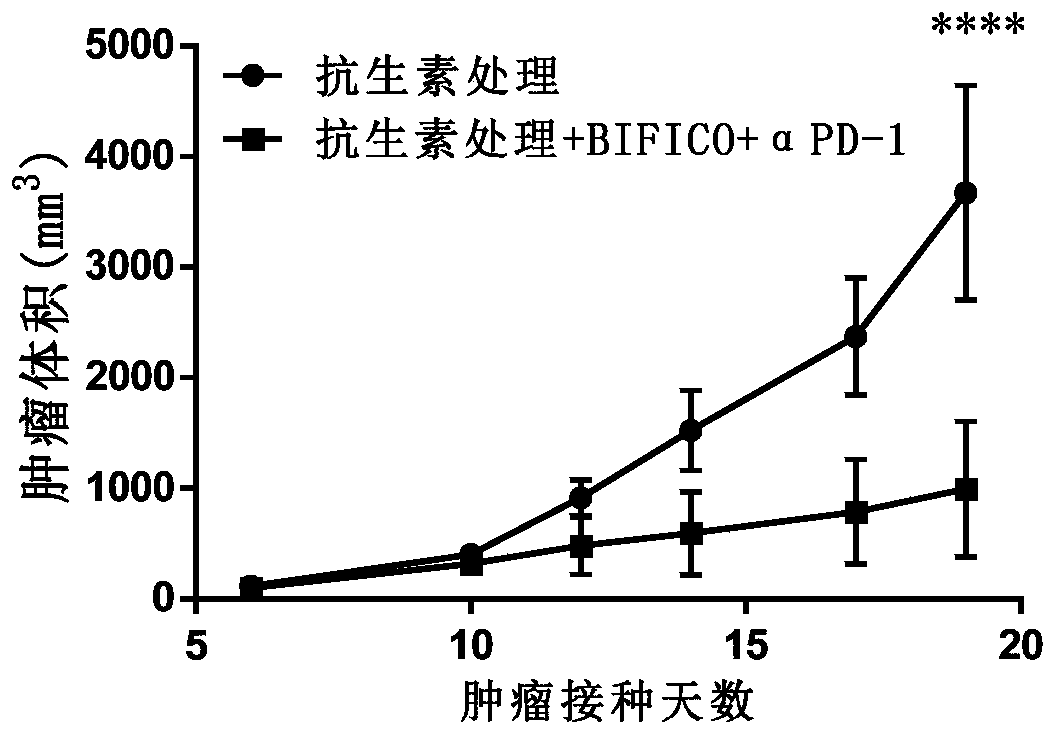
[0076] Twenty-four C57BL / 6J mice at 6-8 weeks were randomly divided into two groups according to body weight after one week of acclimatization, with 12 mice in each group, respectively: group 1 (antibiotic treatment, blank, i.p.), group 2 (antibiotic treatment , BIFICO (p.o.+anti-mPD-1, 10mg / kg, i.p.). Antibiotic treatment is to use broad-spectrum antibiotic Ampicillin (1mg / mL) + colistin (1mg / mL) + streptomycin (5mg / mL) drinking water administration for 5 days .Group 2 began to administer BIFICO freeze-dried samples after antibiotic treatment, at a concentration of 1.0×10 8 CFU / head / day, after continuous gavage for 2 weeks, all groups were inoculated with MC38 tumor cells resuspended in PBS at a concentration of 1×10 7 per mL, inoculated subcutaneously on the right flank of the experimental animals, and the inoculation dose was 100 μL / only. Anti-mPD-1 was injected on the 4th day after tumor cell inoculation, and was injected every 4 days for a total of 4 injections.
[0077...
Embodiment 3
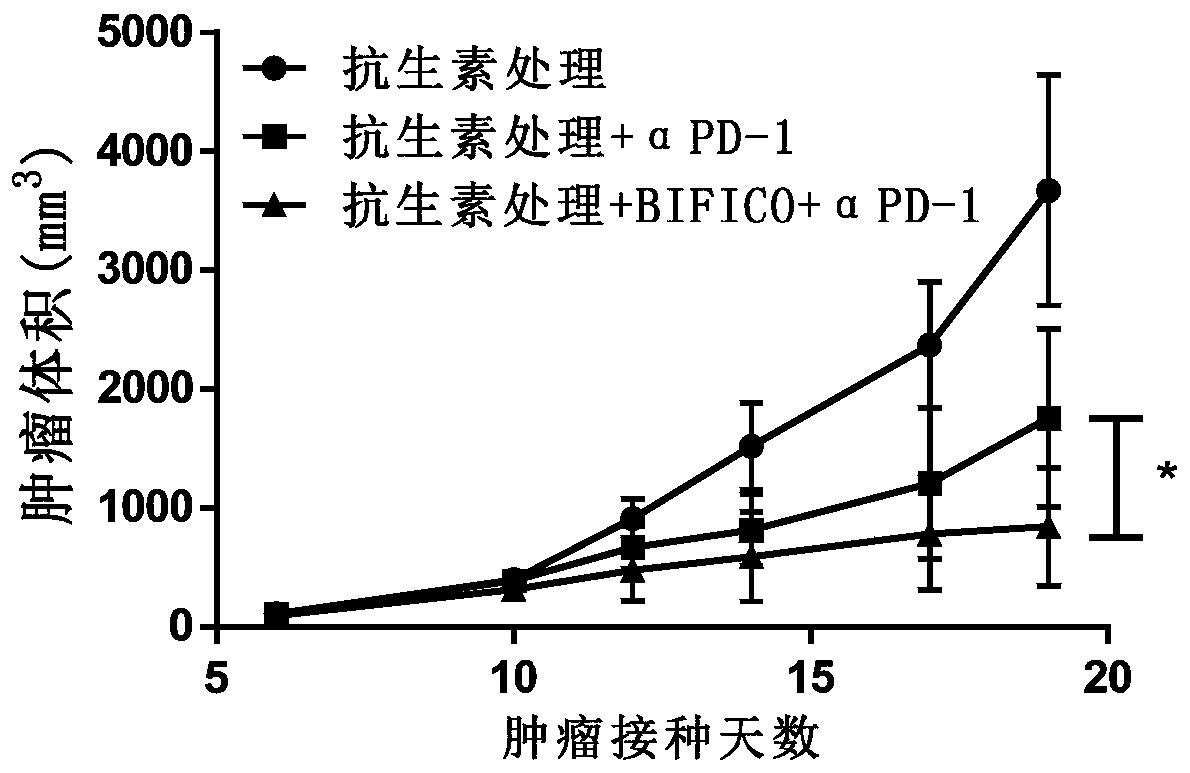
[0080] 36 C57BL / 6J mice at 6-8 weeks were randomly divided into 3 groups according to body weight after one week of acclimatization, 12 mice in each group, respectively: group 1 (antibiotic treatment, blank, i.p.), group 2 (antibiotic treatment , anti-mPD-1, 10mg / kg, i.p.), group 3 (antibiotic treatment, BIFICO, p.o.+anti-mPD-1, 10mg / kg, i.p.). Antibiotic treatment is the use of broad-spectrum antibiotics Ampicillin (1mg / mL) + colistin (1mg / mL) + streptomycin (5mg / mL) drinking water administration for 5 days. The third group of mice began to be given BIFICO freeze-dried samples after antibiotic treatment, with a concentration of 1.0×10 8 CFU / head / day, after continuous gavage for 2 weeks, all groups were inoculated with MC38 tumor cells resuspended in PBS at a concentration of 1×10 7 per mL, inoculated subcutaneously on the right flank of the experimental animals, and the inoculation dose was 100 μL / only. Anti-mPD-1 was injected on the 4th day after tumor cell inoculation, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com