A kind of chitosan hydrogel preparation and preparation method of dihydromyricetin
A technology of dihydromyricetin and gel preparation, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., to achieve improved tumor-inhibiting ability, good biocompatibility, and release effect Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] In another specific embodiment of the present invention, the preparation method of above-mentioned chitosan hydrogel is provided, comprising the following steps:
[0043] (1) Configure a certain concentration of chitosan solution and malic acid solution, add malic acid solution dropwise to the chitosan solution, use a vortex mixer to mix and then place it in a constant temperature water bath to heat and dissolve, and stir continuously during the heating process;
[0044] (2) After the sample in step (1) is fully dissolved, put it into a centrifuge and centrifuge at a constant speed;
[0045] (3) Add Tween-80 solution containing saturated dihydromyricetin to the sample after centrifugation, place it again in a constant temperature water bath for heating in a water bath, and keep stirring during the heating process;
[0046] (4) After the samples in step (3) are fully mixed, centrifuge again to obtain the chitosan hydrogel preparation of the dihydromyricetin.
[0047] In a...
Embodiment 1
[0052] Example 1 Cell lethality experiment
[0053] Collect normal liver cells HL-7702 and liver cancer cells HepG2 in the logarithmic growth phase at 2×10 5 Cells were seeded in 96-well plates, 200 μL per well. After culturing for 2 hours until the cells adhered to the wall, 1 mg of dihydromyricetin-based chitosan hydrogel carrier was added to the culture medium. After 48 hours of culture, 20 μL MTT was added to each well, and the culture was terminated after continuing to culture for 4 hours. Add 150 μL of DMSO to each well, shake on a shaker at a low speed, detect the OD value at 490 nm with a microplate reader, and further calculate the survival rate of the cells. The results are shown in the attached Figure 5 , 6 shown.
Embodiment 2
[0054] Example 2 In vitro release simulation experiment
[0055] The prepared dihydromyricetin chitosan gel was placed in a dialysis bag, and 30% ethanol aqueous solution was selected as the release medium, and the gels were released at 1, 2, 3, 4, 5, 7, 9, 12, 16, 23 , 28, 33, 38, and 47h time points, take samples to measure the drug concentration in the release medium, and calculate the drug release amount.
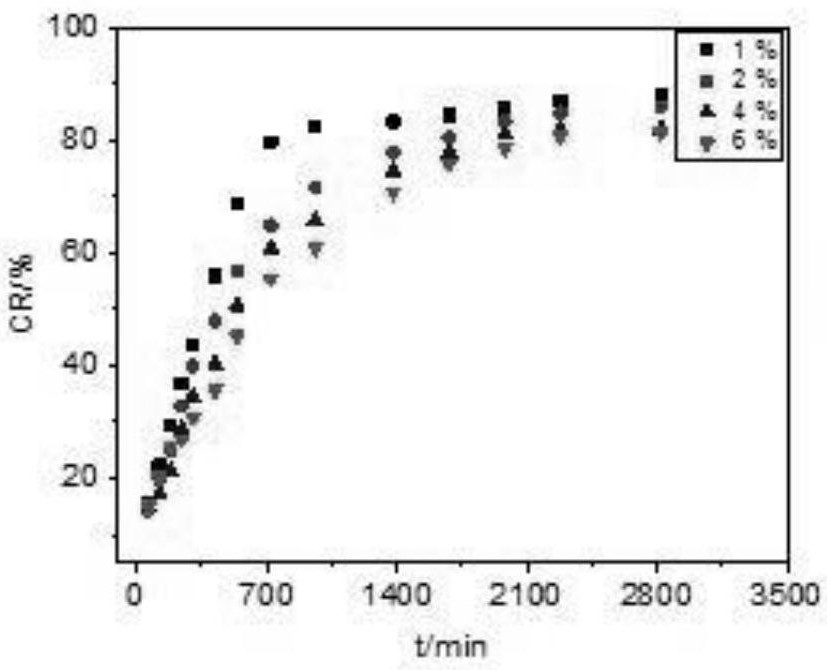
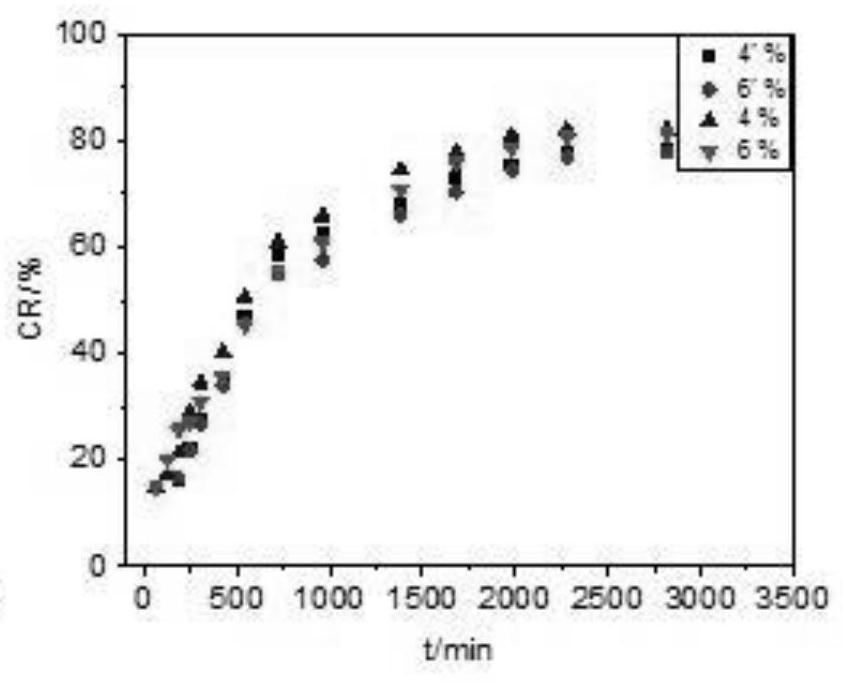
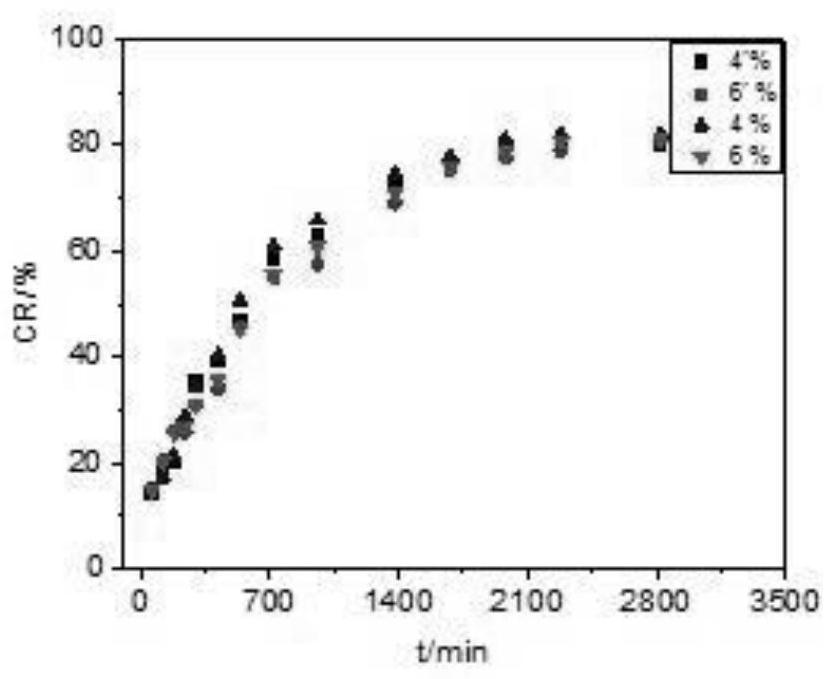
[0056] 1. In vitro drug release results of samples prepared with different chitosan contents
[0057] The results are attached figure 1 As shown, when the fixed Tween-80 content is 1.5g, the mass percentage of chitosan is 1%, 2%, 4%, and 6% of the four groups of samples. hormone release experiments. Among them, the chitosan with 1% content appeared relatively fast release, which showed a certain burst release phenomenon in the first 700min, and the release behavior of 2%, 4%, and 6% samples was relatively slow throughout the release process. It has a certain sustain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com