Preparation method and application of ferrous valine
A technology of ferrous valine and valine, which is applied in the field of preparation of amino acid chelates, can solve problems such as the preparation method and application of ferrous valine that have not been reported, and achieve improved animal growth performance and good application prospects , the effect of reducing the amount of addition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A method for preparing ferrous valine, comprising the following steps: turning on stirring, adding 239.1Kg of valine with a purity of 98% to 2.2t water, turning on the stirring, and then adding 109.2Kg of sodium carbonate with a purity of 98%, After stirring until there are no more bubbles, add 171.7Kg of ferrous sulfate monohydrate with a purity of 96% and raise the temperature to 30°C, react for 2h. After the reaction, press filter and flash dry to obtain 301.8Kg of product.
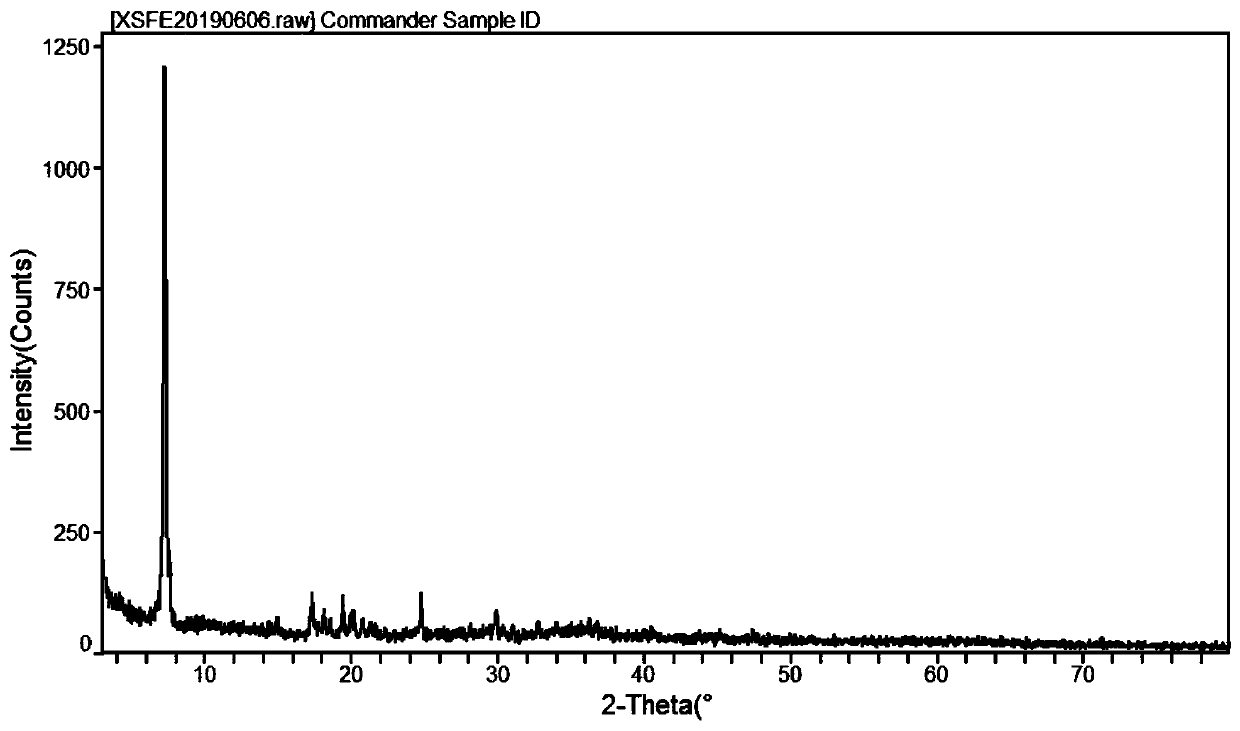
[0047] In this example, the content of valine in the ferrous valine product is 73.7%, and Fe 2+ 16.0%, Fe 3+ It is 1.7%, the water loss rate at 180°C is 5.7%, the water loss rate at 104°C is 2.4%, that is, the purity is 97.1%, and the yield is 98.7% based on total iron, which is the molar ratio of valine to ferrous iron. About 2:1, the molecular formula is Fe(C 5 H 10 NO 2 ) 2 ·H 2 O. The powder diffraction pattern of ferrous valine obtained in this example is shown in figure 1 .
Embodiment 2
[0049] A method for preparing ferrous valine, comprising the following steps: turning on stirring, adding 239.1Kg of valine with a purity of 98% to 2.3t water, turning on the stirring, and adding 143.9Kg of potassium carbonate with a purity of 98%. After stirring until there are no more bubbles, add 249.4Kg of ferrous sulfate pentahydrate with a purity of 98% and raise the temperature to 40°C, add 0.37Kg of 10 mesh reduced iron powder, and react for 1.5h. After the reaction is complete, cool to Below 40°C, add 190L of ethanol to crystallize, centrifuge and dry in the oven to obtain 299.6Kg of product.
[0050] The content of valine in ferrous valine products is 74.6%, Fe 2+ 17.8%, Fe 3+ The water loss rate at 180°C is 5.8%, and the water loss rate at 104°C is 1.0%. The purity is 98.3%. The yield is 96.2% based on valine, which is the mole of valine and ferrous iron. The ratio is about 2:1, and the molecular formula is Fe(C 5 H 10 NO 2 ) 2 ·H 2 O.
Embodiment 3
[0052] A method for preparing ferrous valine, comprising the following steps: turning on stirring, adding 239.1Kg of valine with a purity of 98% to 2.0t water, turning on the stirring, and adding 173.1Kg of sodium bicarbonate with a purity of 98% After stirring until there are no more bubbles, add 280.9Kg of ferrous sulfate heptahydrate with a purity of 99% and increase the temperature to 45℃, add 0.28Kg of 40 mesh reduced iron powder, and react for 1.8h. After the reaction is completed, the reaction is completed and then cooled Below 40°C, add 420L of isopropanol and crystallize, centrifuge and flash dry to obtain 305.1Kg of product.
[0053] The content of valine in ferrous valine products is 74.1%, Fe 2+ 17.7%, Fe 3+ 0.1%, the water loss rate at 180°C is 5.7%, the water loss rate at 104°C is 2.0%, that is, the purity is 97.7%, and the yield is 97.4% based on valine, which is the mole of valine and ferrous iron The ratio is about 2:1, and the molecular formula is Fe(C 5 H 10 NO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com