A kind of preparation method and application of zinc valine
A technology of zinc valine and valine, which is applied in the field of preparation of amino acid chelates, can solve the problems of long process cycle, high pollution of waste liquid, difficult purification and the like, and achieves high product purity, no by-products, and chemical reaction. high speed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A method for preparing zinc valine, comprising the following steps: start stirring, add 478.1Kg of valine with a purity of 98% to 3.0t of water for dissolution, heat up to 40°C, and slowly add an active oxidation agent with a zinc content of 77.3%. 169.2Kg of zinc was reacted for 2.5 hours. After the reaction, the reaction system was cooled to below 40°C, crystallized, separated by centrifugal filtration, washed with water three times, and dried in an oven to obtain 612.7Kg of zinc valine.
[0039] In the present embodiment, it is measured that the valine content in the valine zinc product is 72.0%, Zn 2+ The water loss rate is 20.3%, the water loss rate is 5.6% at 180°C, and the water loss rate is 2.0% at 104°C, that is, the purity is 97.9%, and the yield is 95.0% based on valine, that is, the molar ratio of valine to zinc About 2:1, the molecular formula is Zn(C 5 h 10 NO 2 ) 2 ·H 2 O.
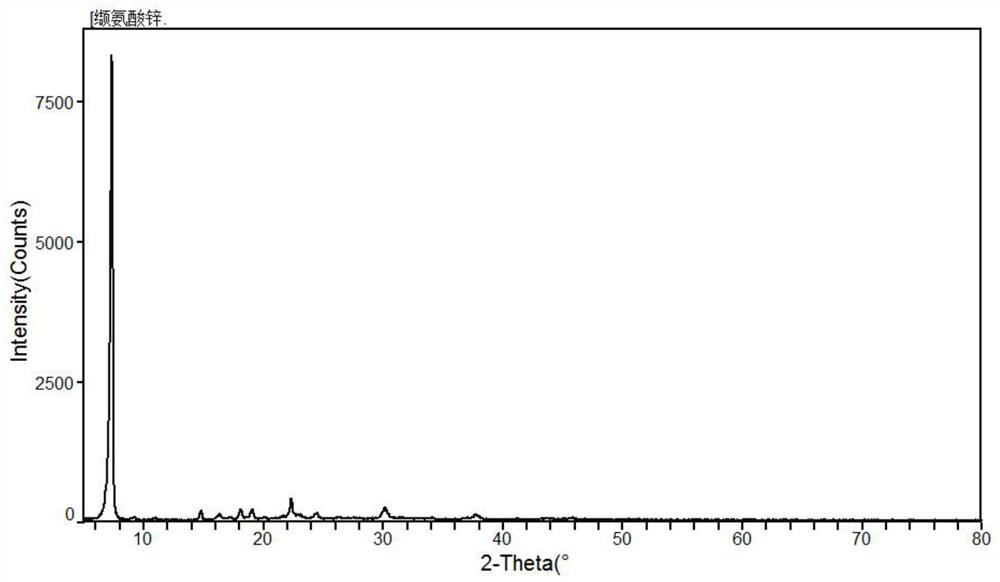
[0040] The XRD figure of the zinc valine prepared in the present embodiment...
Embodiment 2
[0042] A method for preparing zinc valine, comprising the following steps: start stirring, add 478.1Kg of valine with a purity of 98% to 5.4t of water for dissolution, then heat up to 55°C, slowly add zinc carbonate with a zinc content of 49.5% 264.0Kg was reacted for 2.5 hours. After the reaction, the reaction system was cooled to below 40°C, 110L of acetone was added to crystallize, separated by centrifugal filtration, washed with water three times, and dried in an oven to obtain 609.8Kg of zinc valine.
[0043] In the present embodiment, it is measured that the valine content in the valine zinc product is 70.9%, Zn 2+ The water loss rate is 20.0%, the water loss rate is 5.5% at 180°C, and the water loss rate is 2.5% at 104°C, that is, the purity is 96.4%, and the yield is 93.1% based on valine, that is, the molar ratio of valine to zinc About 2:1, the molecular formula is Zn(C 5 h 10 NO 2 ) 2 ·H 2 O.
Embodiment 3
[0045] A method for preparing zinc valine, comprising the following steps: start stirring, add 459.0 Kg of valine with a purity of 98% to 2.1 t of water for dissolution, heat up to 68°C, and slowly add a basic formula with a zinc content of 58.4%. 67.2Kg of zinc carbonate was reacted for 0.2h, and then 156.8Kg of basic zinc carbonate with a zinc content of 58.4% was added to react for 2h. After the reaction, the reaction system was cooled to below 40°C to crystallize, and 150L of ethanol was added to crystallize. After separation by centrifugal filtration, water Washed 3 times, dried in an oven to obtain 633.8Kg of zinc valine.
[0046] In the present embodiment, it is measured that the valine content in the valine zinc product is 72.6%, Zn 2+ The water loss rate is 20.5%, the water loss rate is 5.6% at 180°C, and the water loss rate is 1.2% at 104°C, that is, the purity is 98.7%, and the yield is 96.9% based on zinc, that is, the molar ratio of valine to zinc is about 2:1, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com