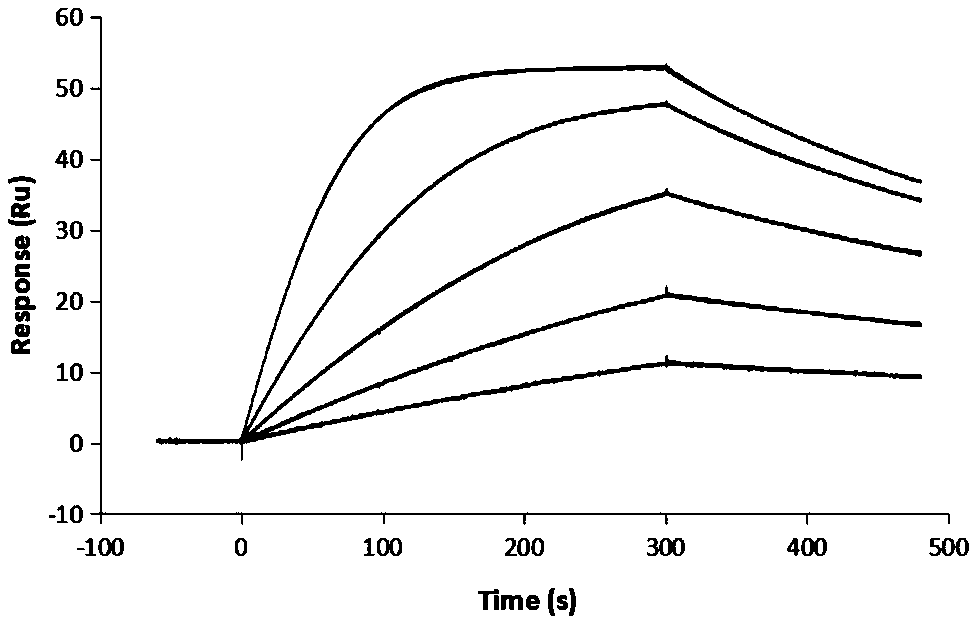
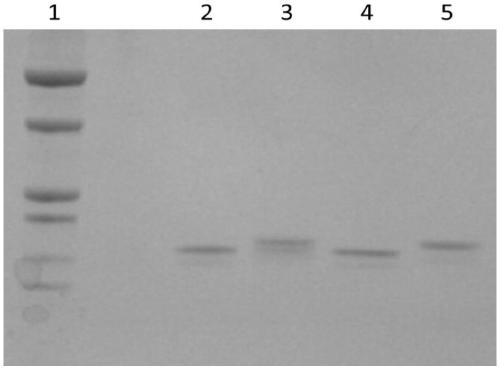
Novel anti-human serum albumin antibody fragment, preparation method and application
An anti-human serum and albumin technology, applied in the field of biomedicine, can solve the problems of low expression level, difficult purification work, large molecular weight of human serum albumin fusion protein, etc., and achieve high affinity, high stability, and good specificity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Construction of anti-human serum albumin single domain antibody library
[0028] Two Xinjiang Bactrian camels were immunized with human serum albumin mixed with Freund's adjuvant, once a week for a total of seven times. After the immunization, the peripheral blood cells of camels were extracted, and the lymphocytes were frozen in dry ice and sent to Nanjing Jaxes Biotechnology Co., Ltd. for the extraction and separation of single domain antibody fragments. The steps of extraction and isolation of single-domain antibody fragments are as follows: extract total RNA, synthesize cDNA by reverse transcription, amplify single-domain antibody fragments by nested PCR, digest with restriction endonucleases, ligate into phage display vectors, and electroporate into competent cells. Two independent single-domain antibody phage display libraries against human serum albumin were successfully constructed. The library capacity was determined by counting the number of single...
Embodiment 2
[0029] Example 2: Screening process of single domain antibody against human serum albumin
[0030] Coupling human serum albumin on the ELISA plate overnight, adding the phage library after blocking with blocking solution, washing with PBST for several times, using TEA eluent to dissociate the phage that specifically binds to human serum albumin, and use it for infection Escherichia coli cells in the logarithmic growth phase, and the phages were expanded for the next round of screening. Each library was enriched by more than 500 times after three rounds of Bio-panning, achieving the purpose of using phage display technology to screen the specific antibody binding to human serum albumin in the antibody library.
Embodiment 3
[0031] Embodiment 3: use the enzyme-linked immunoassay method (ELISA) of phage to screen specific single positive clone
[0032]Randomly select 600 single colonies from the two libraries to inoculate and culture. After growing to the logarithmic phase, IPTG induces expression. After the bacteria are collected by centrifugation, the crude antibody is obtained from the periplasm by osmotic shock method, and the enzyme coated with human serum albumin is added. Incubate in the standard plate, wash the plate with PBST, use mouse Anti-HA tag antibody as primary antibody, goat anti-mouse alkaline phosphatase conjugate antibody (goat anti-mouse alkaline phosphatase conjugate) as secondary antibody, add alkaline phosphatase Develop color and read the absorbance value, and judge the sample wells with OD value more than 3 times higher than the control wells as positive control wells. All positive clones were cultured, plasmids were extracted and sequenced to obtain multiple single-domain...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap