Application of eight-membered oxygen bridge heterocyclic compound as synergist of bee selective insecticide
A compound and insecticide technology, applied in the field of pesticides, can solve the problem that honeybees do not show selectivity, increase the insecticidal activity of imidacloprid neonicotinoid insecticides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
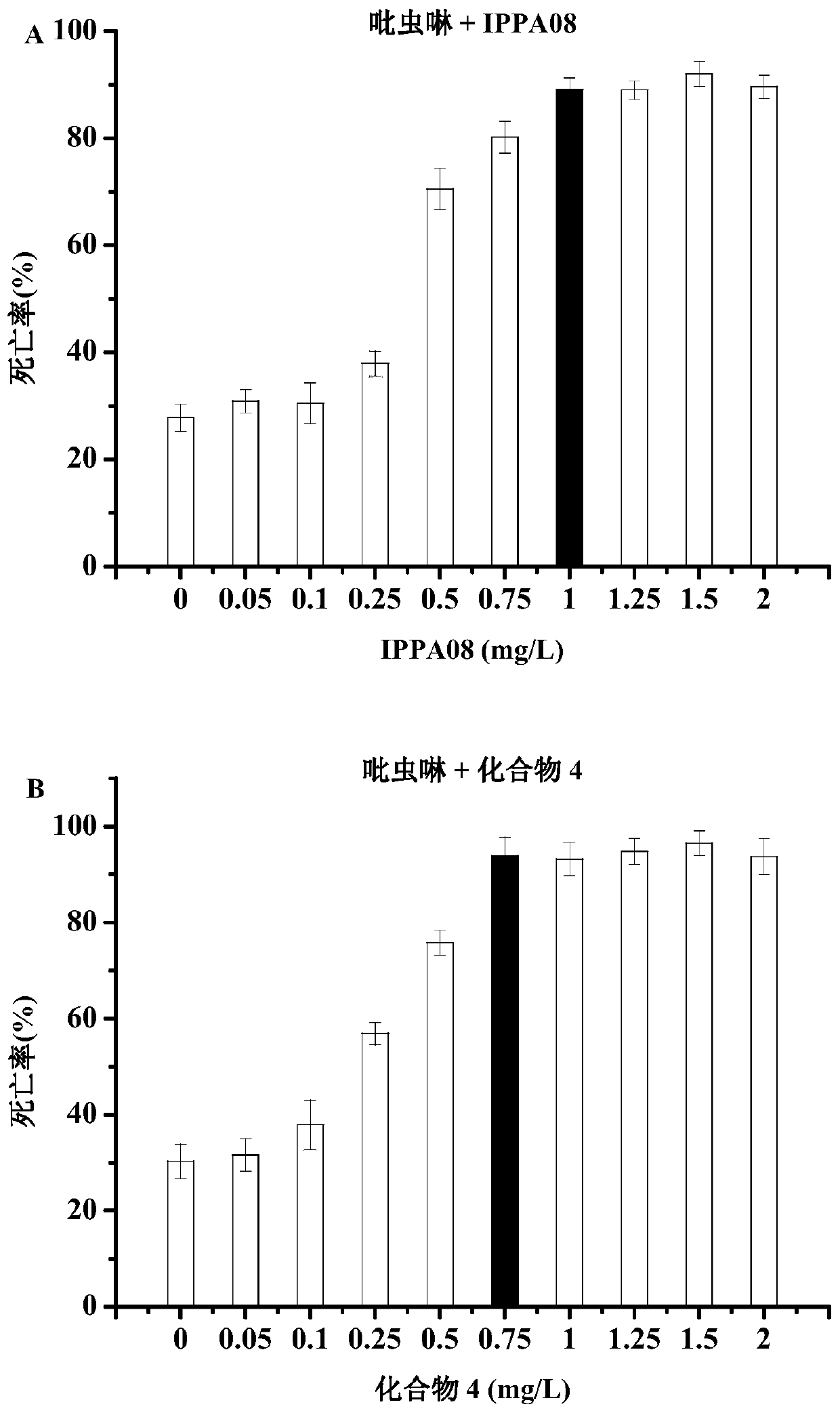
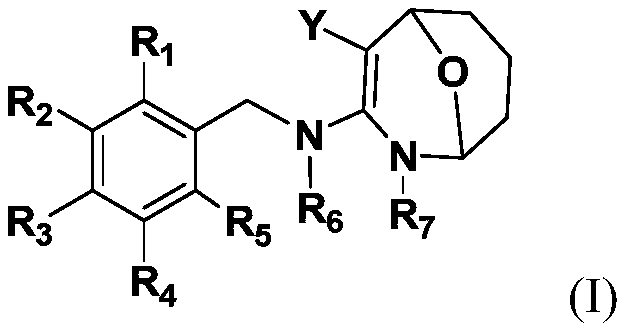
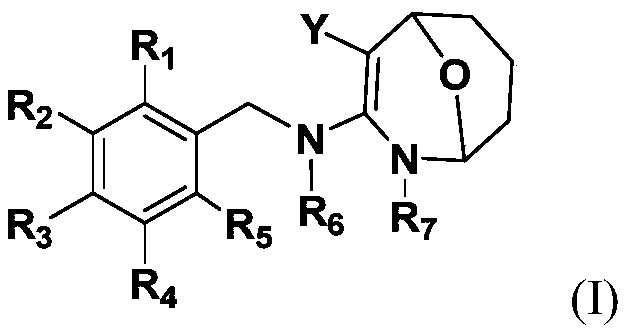
Image
Examples
Embodiment 1
[0066] Example 1: 1-(4-methylphenyl)-10-nitro-1,2,3,5,6,7,8,9-octahydro-5,9-epoxyimidazo[1, Synthesis of 2-a] azacyclooctene
[0067]
[0068] At room temperature, 1-(4-methylphenyl)-2-(nitromethylene) imidazolidine (1.165g, 5mmol) and 50% glutaraldehyde aqueous solution (1.2g, 6mmol) were dissolved in 20mL without Add a catalytic amount of concentrated hydrochloric acid (0.1 mL) to water acetonitrile and stir to react, followed by TLC. After the reaction, saturated NaHCO 3 The pH of the aqueous solution was adjusted to neutral, extracted with dichloromethane (20mL×3), the organic phase was collected and washed with anhydrous Na 2 SO 4 Drying, concentration, and separation by column chromatography yielded a light yellow powdery pure product with a yield of 80%; m.p.122.9-124.1°C; 1 H NMR (400MHz, CDCl 3 )δ7.18(dd,J=15.6,7.8Hz,4H),5.37–5.29(m,1H),4.96(d,J=14.8Hz,1H),4.83(d,J=14.8Hz,1H), 3.75–3.64(m,1H),3.63–3.45(m,4H),2.34(s,3H),2.16(d,J=13.6Hz,1H),1.94–1.83(m,2H),1.64...
Embodiment 2
[0069] Example 2: 1-(4-isopropylphenyl)-10-nitro-1,2,3,5,6,7,8,9-octahydro-5,9-epoxyimidazo[1 ,Synthesis of 2-a]azacyclooctene
[0070]
[0071] At room temperature, 1-(4-isopropylphenyl)-2-(nitromethylene) imidazolidine (1.305g, 5mmol) and 50% glutaraldehyde aqueous solution (1.2g, 6mmol) were dissolved in 20mL In anhydrous acetonitrile, a catalytic amount of concentrated hydrochloric acid (0.1 mL) was added to stir the reaction, followed by TLC. After the reaction, saturated NaHCO 3 The pH of the aqueous solution was adjusted to neutral, extracted with dichloromethane (20mL×3), the organic phase was collected and washed with anhydrous Na 2 SO 4 Drying, concentration, and separation by column chromatography gave a pure yellow powder with a yield of 83%; m.p.140.2-141.3°C; 1 H NMR (400MHz, DMSO-d 6 )δ7.34–7.14(m,4H),5.07-5.03(m,2H),4.96(d,J=15.0Hz,1H),4.75(d,J=15.0Hz,1H),3.76–3.54(m ,4H),2.94–2.80(m,1H),1.83–1.44(m,6H),1.19(d,J=6.8Hz,6H)ppm. 13 C NMR (101MHz, DMSO-d 6...
Embodiment 3
[0072] Example 3: 1-(4-ethylphenyl)-10-nitro-1,2,3,5,6,7,8,9-octahydro-5,9-epoxyimidazo[1, Synthesis of 2-a] azacyclooctene
[0073]
[0074] At room temperature, 1-(4-ethylphenyl)-2-(nitromethylene) imidazolidine (1.235g, 5mmol) and 50% glutaraldehyde aqueous solution (1.2g, 6mmol) were dissolved in 20mL without Add a catalytic amount of concentrated hydrochloric acid (0.1 mL) to water acetonitrile and stir to react, followed by TLC. After the reaction, saturated NaHCO 3 The pH of the aqueous solution was adjusted to neutral, extracted with dichloromethane (20mL×3), the organic phase was collected and washed with anhydrous Na 2 SO 4 Drying, concentration, and separation by column chromatography gave a pure yellow powder with a yield of 90%; m.p.118.9-119.1°C; 1 H NMR (400MHz, CDCl 3 )δ7.18(dd,J=15.6,7.8Hz,4H),5.37–5.29(m,1H),4.96(d,J=14.8Hz,1H),4.83(d,J=14.8Hz,1H), 3.75–3.64(m,1H),3.63–3.45(m,4H),2.34(s,3H),2.16(d,J=13.6Hz,1H),1.94–1.83(m,4H),1.64–1.62( m,3H)ppm. 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com