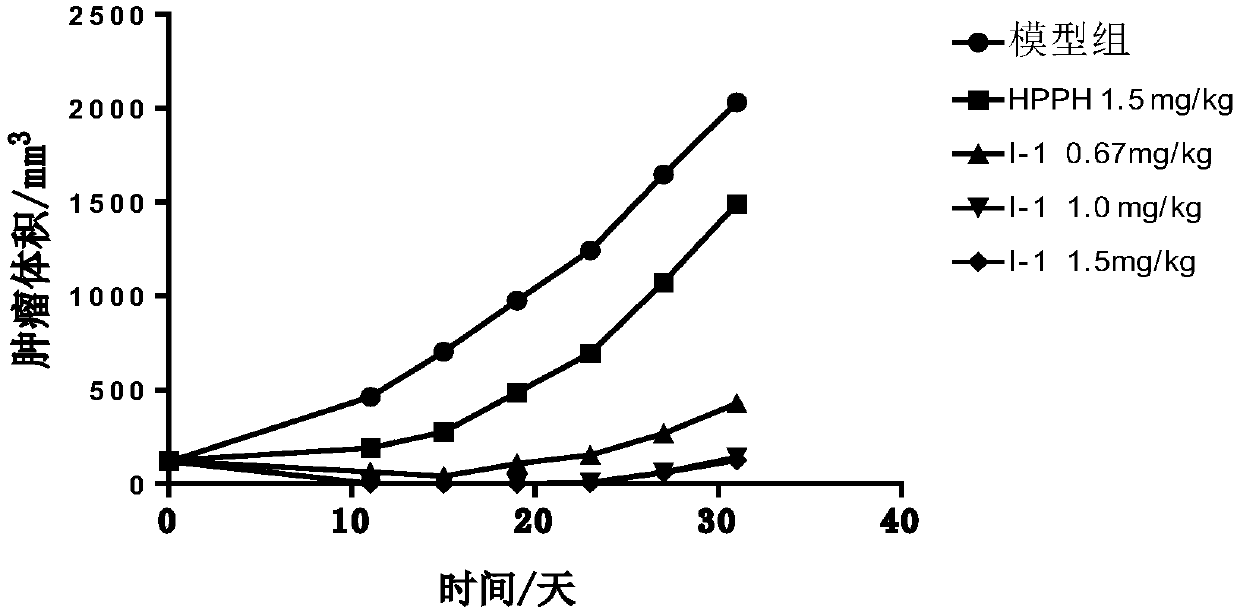
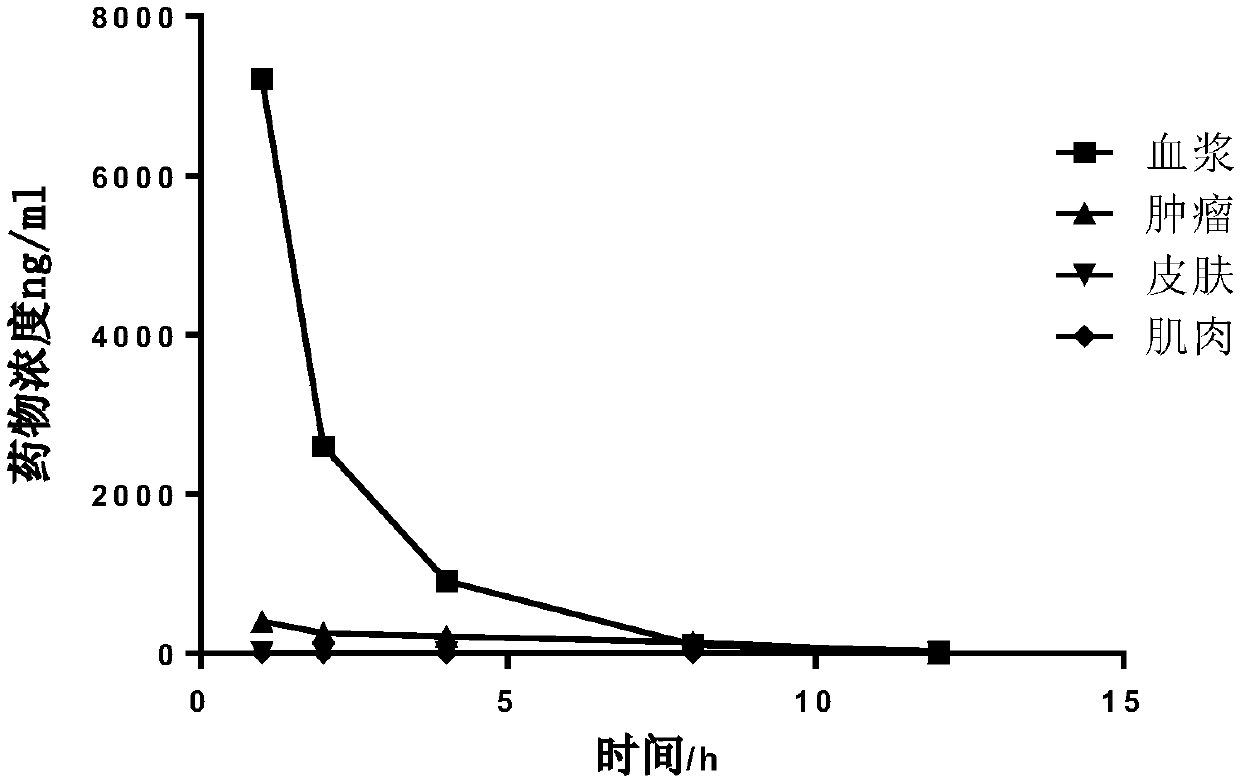
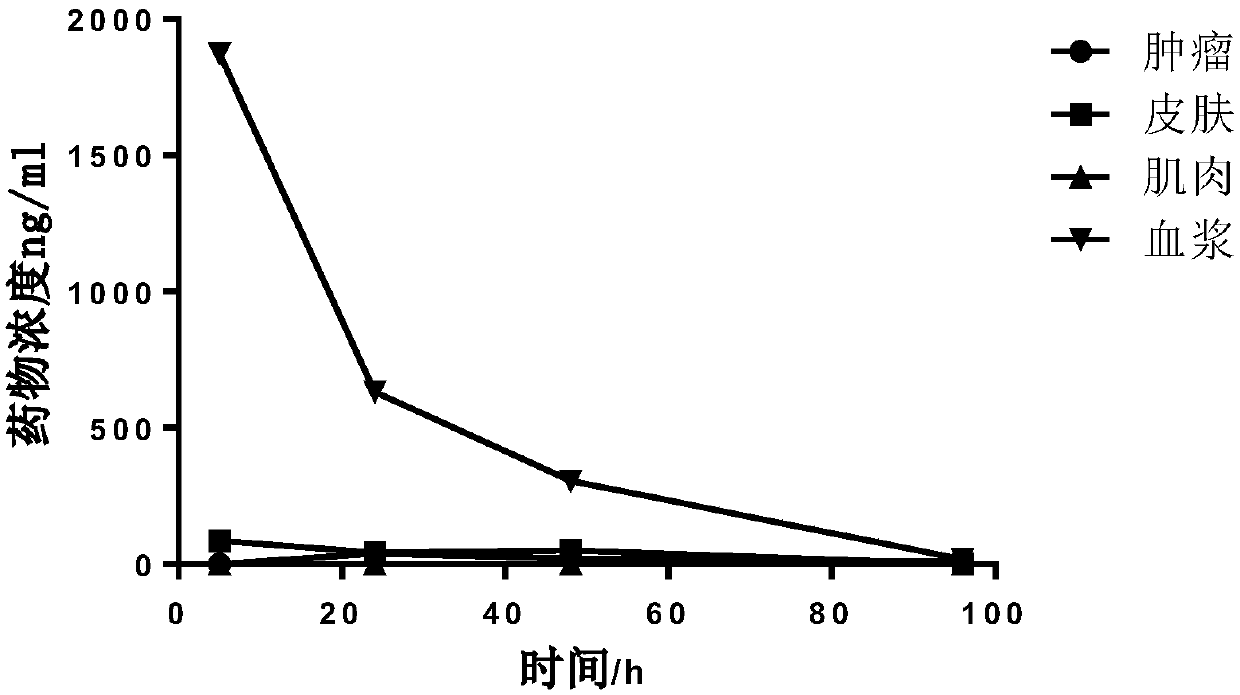
Bacterial chlorophyll derivative and preparation method thereof
A halogen and drug technology, which is applied in the field of its preparation and bacteriochlorophyll derivatives, can solve the problems of long light avoidance period and slow metabolism of photosensitizers, etc., and achieve the effect of short light avoidance period, good chemical stability and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] The synthesis of embodiment 1 compound (I-1)
[0091]
[0092] The first step: the synthesis of methyl 2-(4-hydroxymethyl)phenoxyacetate (IV-1)
[0093] In a 500mL three-necked round-bottomed flask, add p-hydroxybenzaldehyde (10g, 82mmol), then add 120mL of acetonitrile, stir and dissolve, then add methyl bromoacetate (10mL, 105mmol) and potassium carbonate (15g, 9.2mmol), Stir the reaction at room temperature, and monitor the reaction by TLC. After the reaction, add 150 mL of ethyl acetate to dilute the reaction solution, filter, and concentrate the filtrate under reduced pressure to obtain a yellow oil, which is directly carried out to the next step of reaction;
[0094]The obtained yellow oil was transferred to a 500mL three-necked round-bottomed flask, 100mL of dichloromethane and 50mL of methanol were added, stirred, and under ice-bath cooling, sodium borohydride (3.0g, 79mmol) was slowly added. After the addition, Stir the reaction at room temperature, and mon...
Embodiment 2
[0108] The synthesis of embodiment 2 compound (I-2):
[0109]
[0110]
[0111] The first step: the synthesis of methyl 5-(3-methyl-4-hydroxymethyl)phenoxyvalerate (IV-2)
[0112] In a 500mL three-neck round bottom flask, add 3-methyl-4-hydroxybenzaldehyde (5.0g, 36.7mmol), then add 80mL of acetonitrile, stir to dissolve, then add methyl 5-bromovalerate (8.0g, 41.0mmol) and potassium carbonate (7.0g, 50.6mmol), stirred at 60°C, and monitored by TLC. After the reaction, 150mL of ethyl acetate was added to dilute the reaction solution, filtered, and the filtrate was concentrated under reduced pressure to obtain a yellow oil;
[0113] Transfer the obtained yellow oil into a 500mL three-necked round-bottomed flask, add 100mL of dichloromethane and 50mL of methanol, stir, and slowly add sodium borohydride (0.75g, 2.04mmol) under ice-bath cooling. After the addition, Stir the reaction at room temperature, and monitor the reaction by TLC. After the reaction, quench the reactio...
Embodiment 3
[0127] The synthesis of embodiment 3 compound (I-3):
[0128]
[0129]
[0130] The first step: the synthesis of methyl 2-(3-methyl-(4-hydroxymethyl))phenoxyacetate (IV-3)
[0131] In a 500mL three-neck round bottom flask, add 3-methyl-4 hydroxybenzaldehyde (5.02g, 25.5mmol), then add 80mL of acetonitrile, stir and dissolve, then add methyl bromoacetate (2.5mL, 2.55mmol) and Potassium carbonate (5.0g, 3.61mmol), stirring reaction at room temperature, TLC monitoring reaction, after the reaction, add 150mL ethyl acetate to dilute the reaction solution, filter, and concentrate the filtrate to dryness under reduced pressure to obtain a yellow oil, directly proceed to the next step reaction ;
[0132] Transfer the obtained yellow liquid into a 500mL three-necked round-bottomed flask, add 100mL of dichloromethane and 50mL of methanol, stir, and slowly add sodium borohydride (0.7g, 1.9mmol) under ice-bath cooling. Stir the reaction, TLC monitors the reaction, after the reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| penetration depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com