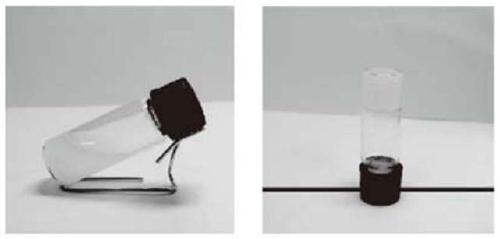
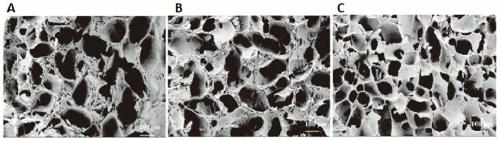
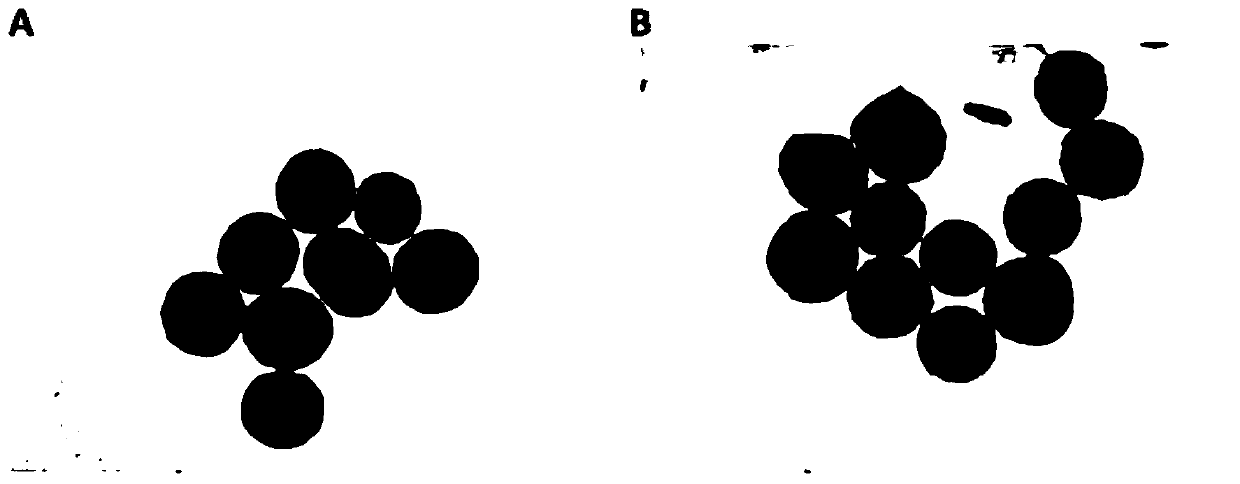
Injectable cartilage repair hydrogel and preparation method thereof
A cartilage repair and hydrogel technology, which is applied in the field of biomedical engineering materials, can solve the problems of increasing patient pain, short life, metal joint allograft rejection, etc., and achieve the effect of enhancing the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] An injectable cartilage repair hydrogel, the hydrogel comprising the following components in parts by weight: 10 parts of methacrylated gelatin (Gel-MA), methacrylated hyaluronic acid (HA-MA) 5 parts, 1 part of nanocellulose (CNC), 0.05 parts of melanin nanoparticles loaded with 2-([1,1-biphenyl]-4-ylcarbamoyl)benzoic acid (SMNP-KGN).
[0044] The preparation method of the injectable cartilage repair hydrogel in this example is as follows: weigh 500 μg SMNP-KGN and dissolve it in 1 mL LPBS, ultrasonically disperse it completely, then weigh 0.1 g Gel-MA, 0.05 g HA-MA and 0.01 g CNC , add the above solution, and stir in a water bath at 50°C for 1 hour, then add 0.05mL of 2% (w / v) phenyl-2,4,6-trimethylbenzoyl lithium phosphonate (LAP), and use a wavelength of A 420nm blue flashlight triggers cross-linking to obtain Gel-MA / HA-MA / CNC / SMNP-KGN hydrogel.
[0045] Among them, the preparation method of Gel-MA is: dissolve 1g of gelatin in 10mL of PBS buffer solution (pH=7.4), ...
Embodiment 2
[0049] An injectable cartilage repair hydrogel, the hydrogel comprising the following components in parts by weight: 5 parts of methacrylated gelatin (Gel-MA), methacrylated hyaluronic acid (HA-MA) 1 part, 1 part of nanocellulose (CNC), 0.01 part of melanin nanoparticles loaded with 2-([1,1-biphenyl]-4-ylcarbamoyl)benzoic acid (SMNP-KGN).
[0050] The preparation method of the injectable cartilage repair hydrogel in this example is as follows: weigh 100 μg SMNP-KGN and dissolve it in 1 mL LPBS, ultrasonically disperse it completely, then weigh 0.05 g Gel-MA, 0.01 g HA-MA and 0.01 g CNC , add the above solution, and stir in a water bath at 50°C for 1 hour, then add 0.05mL of 2% (w / v) phenyl-2,4,6-trimethylbenzoyl lithium phosphonate (LAP), and use a wavelength of A 420nm blue flashlight triggers cross-linking to obtain Gel-MA / HA-MA / CNC / SMNP-KGN hydrogel.
[0051] The preparation methods of Gel-MA, HA-MA, and SMNP-KGN are the same as in Example 1.
Embodiment 3
[0053] An injectable cartilage repair hydrogel, the hydrogel comprising the following components by weight: 15 parts of methacrylated gelatin (Gel-MA), methacrylated hyaluronic acid (HA-MA) 10 parts, 2 parts of nanocellulose (CNC), 0.05 parts of melanin nanoparticles loaded with 2-([1,1-biphenyl]-4-ylcarbamoyl)benzoic acid (SMNP-KGN).
[0054]The preparation method of the injectable cartilage repair hydrogel in this example is as follows: weigh 500 μg SMNP-KGN and dissolve it in 1 mL LPBS, disperse it completely by ultrasonic, then weigh 0.15 g Gel-MA, 0.1 g HA-MA and 0.02 g CNC , add the above solution, and stir in a water bath at 50°C for 1 hour, then add 0.05mL of 2% (w / v) phenyl-2,4,6-trimethylbenzoyl lithium phosphonate (LAP), and use a wavelength of A 420nm blue flashlight triggers cross-linking to obtain Gel-MA / HA-MA / CNC / SMNP-KGN hydrogel.
[0055] The preparation methods of Gel-MA, HA-MA, and SMNP-KGN are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com