Anti-EGFR/PD-1 bispecific antibody
A bispecific antibody, PD-1 technology, applied in the direction of antibodies, specific peptides, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
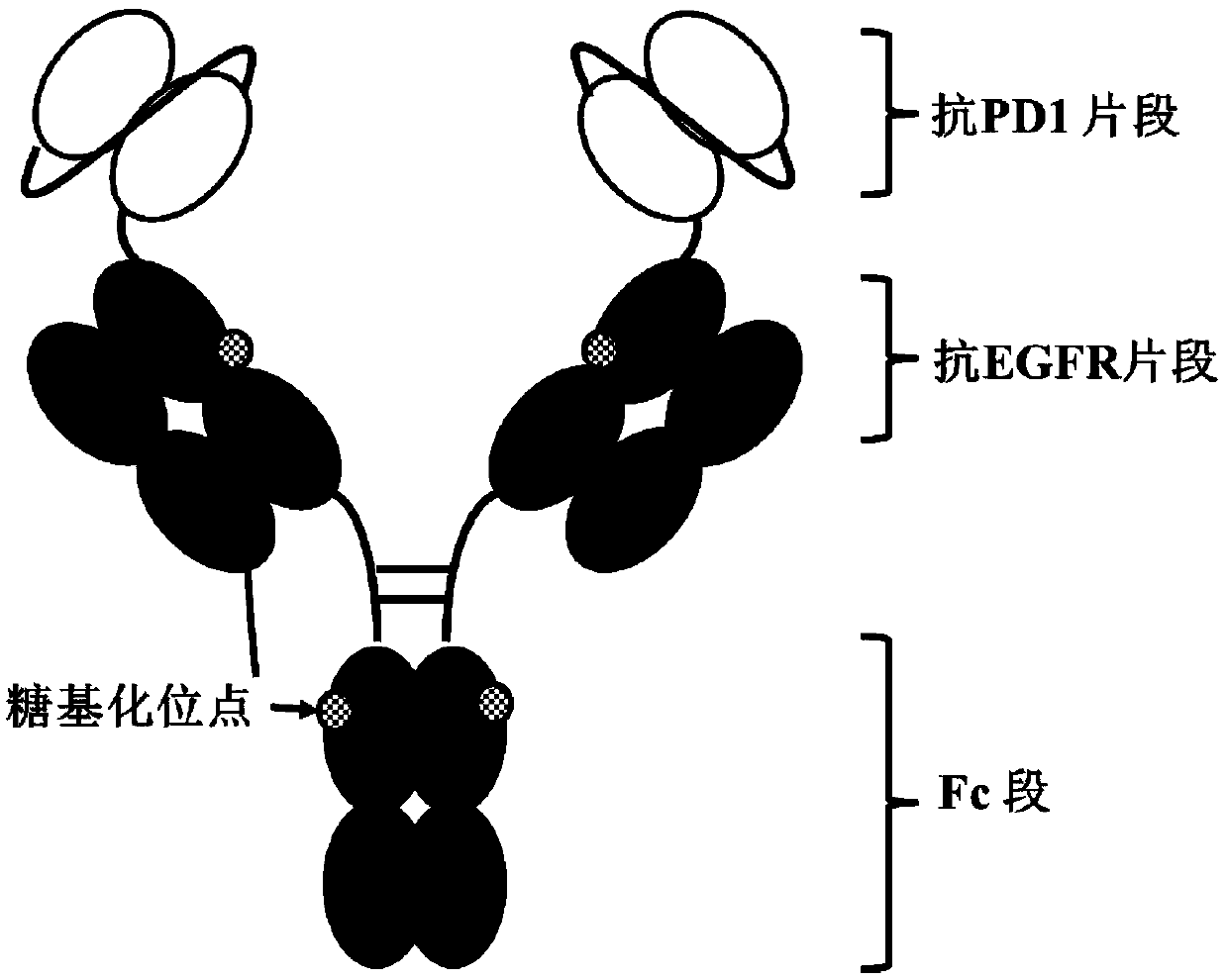
[0122] Example 1 Molecular construction of anti-EGFR / PD-1 bispecific antibody
[0123] In the present invention, the anti-EGFR / PD-1 bispecific antibody is constructed by using scFv and IgG in series, and the specific form is scFv-L2-IgG, such as figure 1 shown. Among them, the scFv adopts the molecular form of VH-L1-VL. The heavy chain variable region VH (SEQ ID NO: 13) and the light chain variable region VL (SEQ ID NO: 14) of the anti-PD-1 monoclonal antibody are connected through L1 (SEQ ID NO: 17) to obtain the anti-PD-1 monoclonal antibody Single-chain antibody fragment scFv of PD-1 (SEQ ID NO: 19). Using L2 (SEQ ID NO: 18) to link the single-chain antibody fragment with the heavy chain of the anti-EGFR monoclonal antibody to obtain the heavy chain of the anti-EGFR / PD-1 bispecific antibody molecule (SEQ ID NO: 20) , the light chain of the EGFR monoclonal antibody (SEQ ID NO: 21) remained unchanged. In order to improve the expression efficiency of antibody molecules in ...
Embodiment 2
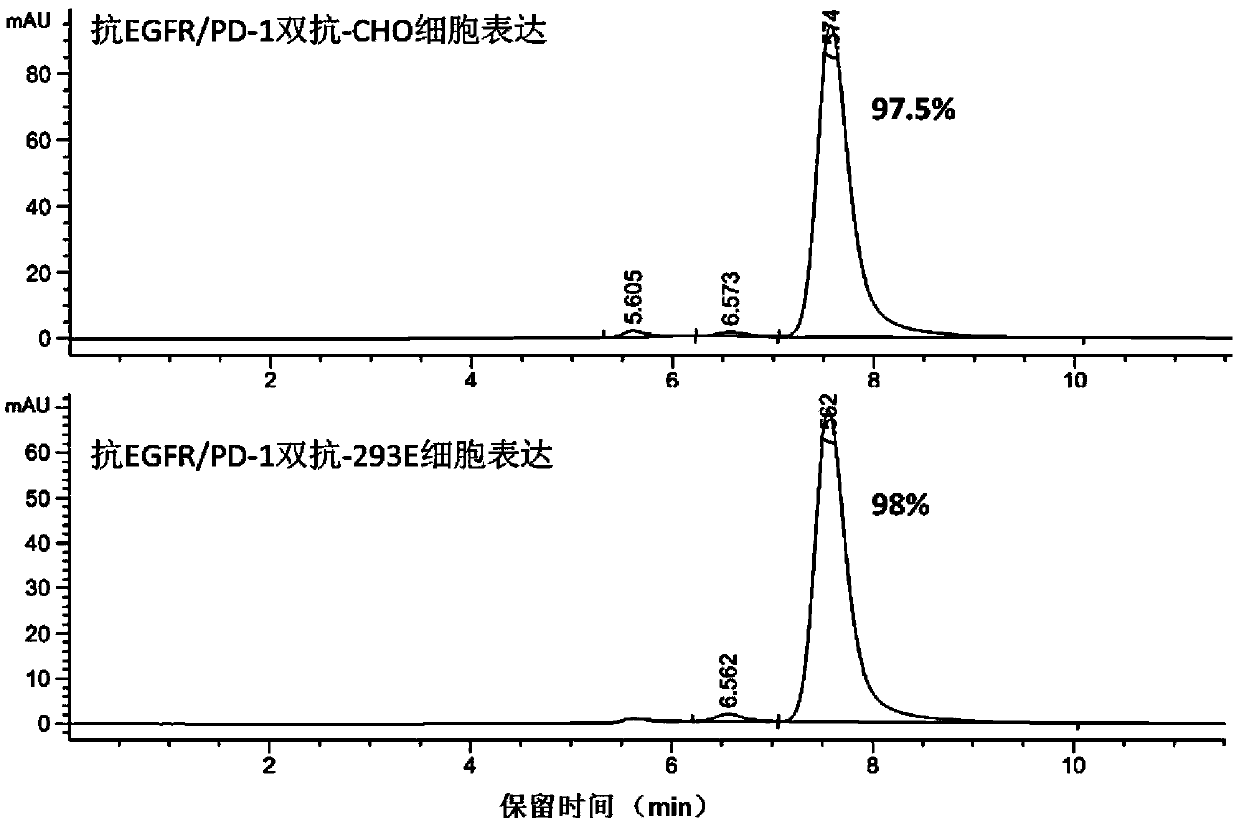
[0124] Example 2 Expression and purification of anti-EGFR / PD-1 bispecific antibody
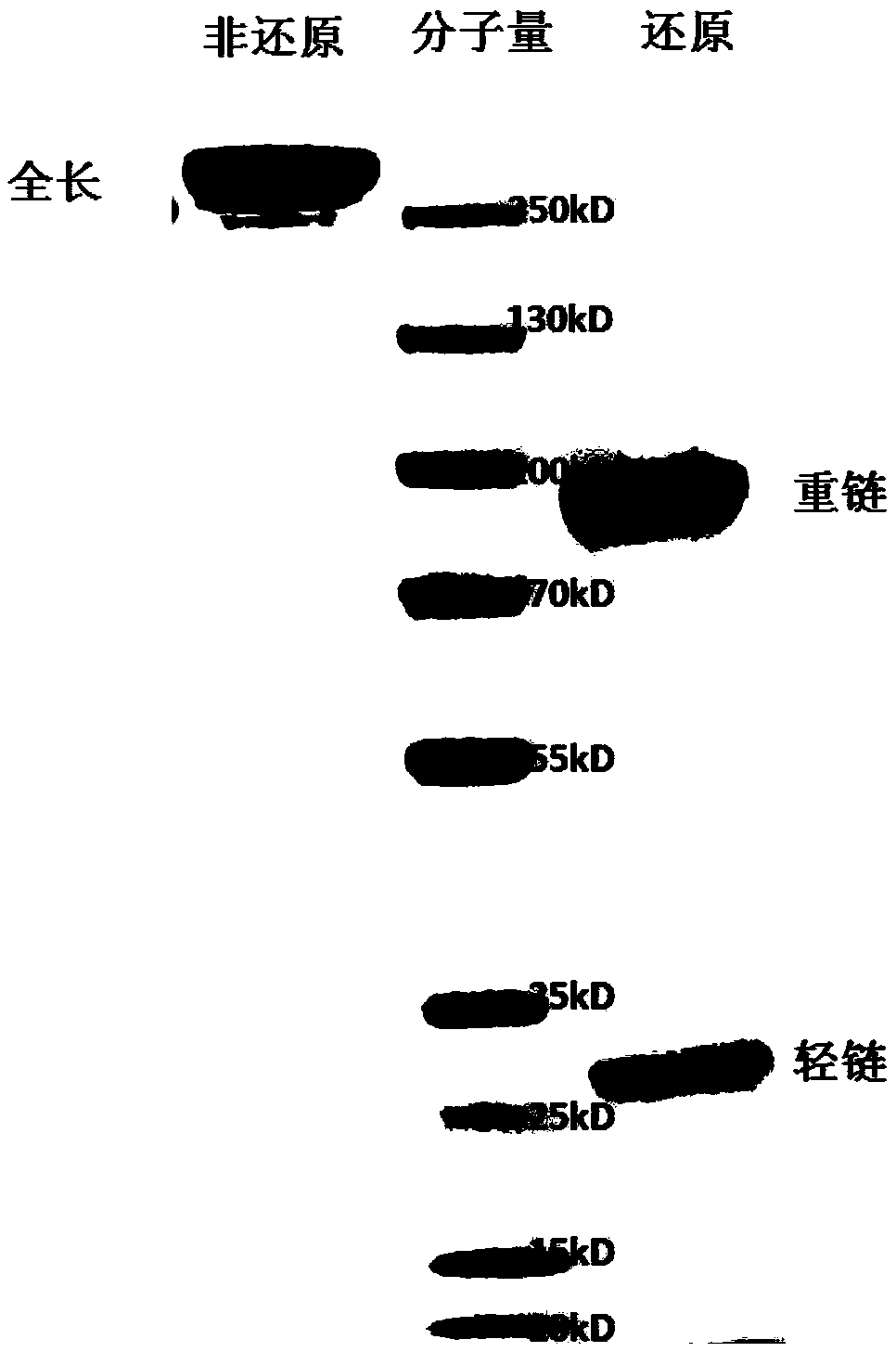
[0125] The DNA fragments of the heavy chain and light chain of the anti-EGFR / PD-1 bispecific antibody were subcloned into the pTT5 vector, and the recombinant plasmids were extracted and co-transfected into CHO cells and / or 293E cells. After 7 days of cell culture, the culture solution was subjected to high-speed centrifugation and vacuum filtration with a microporous membrane, and then loaded onto a HiTrap MabSelectSuRe column, and the protein was eluted in one step with 100mM citric acid, pH3.5 eluent, and the target sample was recovered and dialyzed. solution to PBS. The purified protein was detected by HPLC, such as Figure 2A As shown, the molecular state of the antibody is uniform, and the purity of the monomer reaches more than 97%. Add the reduced protein electrophoresis loading buffer and the non-reduced protein electrophoresis loading buffer respectively, and detect after boiling. ...
Embodiment 3
[0126] Example 3 Determination of affinity of anti-EGFR / PD-1 bispecific antibody to antigen by enzyme-linked immunosorbent assay (ELISA)
[0127] 3.1 ELISA to detect the affinity of anti-EGFR / PD-1 bispecific antibody to EGFR
[0128] Dilute the recombinant EGFR-ECD-Fc protein with coating solution to 3 μg / ml, add 50 μl / well to the microtiter plate, and overnight at 4°C. Wash the plate 3 times with PBST, add 200 μl / well blocking solution, place at 37°C for 1 hour, wash the plate once with PBST and set aside. Dilute the anti-EGFR / PD-1 bispecific antibody to 100 μg / ml with diluent, form 12 concentration gradients (the highest concentration is 100,000 ng / ml, the lowest concentration is 0.02 ng / ml), and add the blocked ELISA plate, 100 μl / well, place at 37°C for 1 hour. Wash the plate three times with PBST, add HRP-labeled mouse anti-human Fab antibody, and place at 37°C for 30 minutes. After washing the plate with PBST for 3 times, pat dry the remaining droplets on absorbent pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com