Green synthesis device of 4-chloroacetoacetate
A green synthesis technology of chloroacetoacetate, applied in the chemical method of reacting liquid and gas medium, preparation of ketene/polyketene, organic chemistry, etc., can solve the problem of raw material loss and reduce by-products The effect of generation, large contact area, and accelerated reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
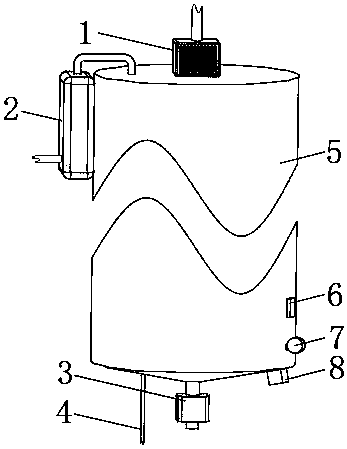
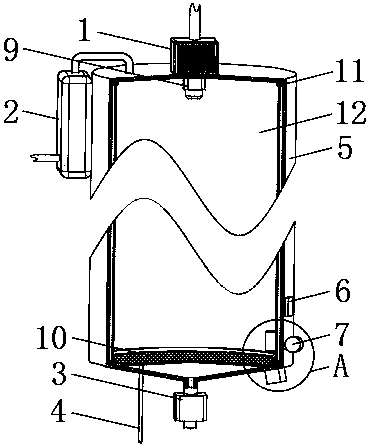
[0033] Example 1: Please refer to Figure 1-3 , the present embodiment discloses a green synthesis device for 4-chloroacetoacetates, comprising a sealed heat-insulating shell 5, the interior of the heat-insulating shell 5 is provided with a conductive cylinder 12, and the upper and lower ends of the conductive cylinder 12 are connected to The upper and lower sides of the inner cavity of the thermal insulation shell 5 are sealed and fixedly connected. An electrostatic generator 8 is provided outside the thermal insulation shell 5. The output end of the electrostatic generator 8 is connected to the side of the conductive cylinder 12 through a cable, and the cable runs through the thermal insulation shell. Body 5.
[0034] The upper part of the inner cavity of the conductive cylinder 12 is provided with an electrostatic sprayer 9, and a precooler 1 is installed on the outside of the conductive cylinder 12. The liquid outlet of the precooler 1 and the liquid inlet of the electrost...
Embodiment 2
[0049] Embodiment two: if figure 2 As shown, this embodiment discloses a green synthesis device for 4-chloroacetoacetates, the structure of which is roughly the same as that of Embodiment 1, the difference being that the lower part of the inner cavity of the conductive cylinder 12 of this embodiment is set There is a grounding grid 10, the side of the grounding grid 10 is fixedly connected to one end of the grounding wire 4, the other end of the grounding wire 4 runs through and extends to the outside of the heat preservation shell 5, and the end of the grounding wire 4 located outside the heat preservation shell 5 is connected to the ground wire connect.
[0050] Preferably, the ground grid 10 is made of metal or alloy.
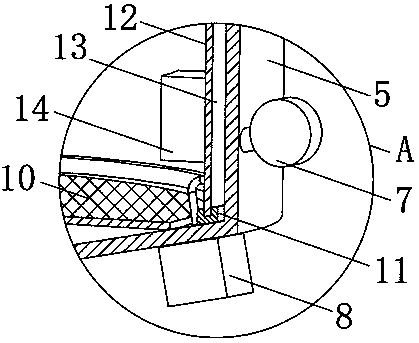
[0051] The working process and principle of this embodiment are: the charged diketene droplet moving to the lower part of the inner cavity of the conductive cylinder 12 contacts the grounding grid 10, and the charge on the diketene droplet passes through t...
Embodiment 3
[0052] Embodiment three: as figure 2 with Figure 4 As shown, this embodiment discloses a green synthesis device for 4-chloroacetoacetates. On the basis of Embodiment 1 or Embodiment 2, an ultrasonic vibrator 14 is fixed on the inside of the conductive cylinder 12 in this embodiment. The input end of the ultrasonic vibrator 14 is electrically connected to the output end of the control switch group 6 .
[0053] Preferably, the upper and lower ends of the conductive cylinder 12 are fixed with rubber rings 11 , and the upper and lower ends of the conductive cylinder 12 are sealed and elastically connected to the upper and lower sides of the inner cavity of the thermal insulation shell 5 through the rubber ring 11 .
[0054] The working process and principle of this embodiment are:
[0055] The operation of the ultrasonic vibrator 14 is controlled by the control switch group 6, and the ultrasonic vibrator 14 causes the conductive cylinder 12 to vibrate. When some diketene dropl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com