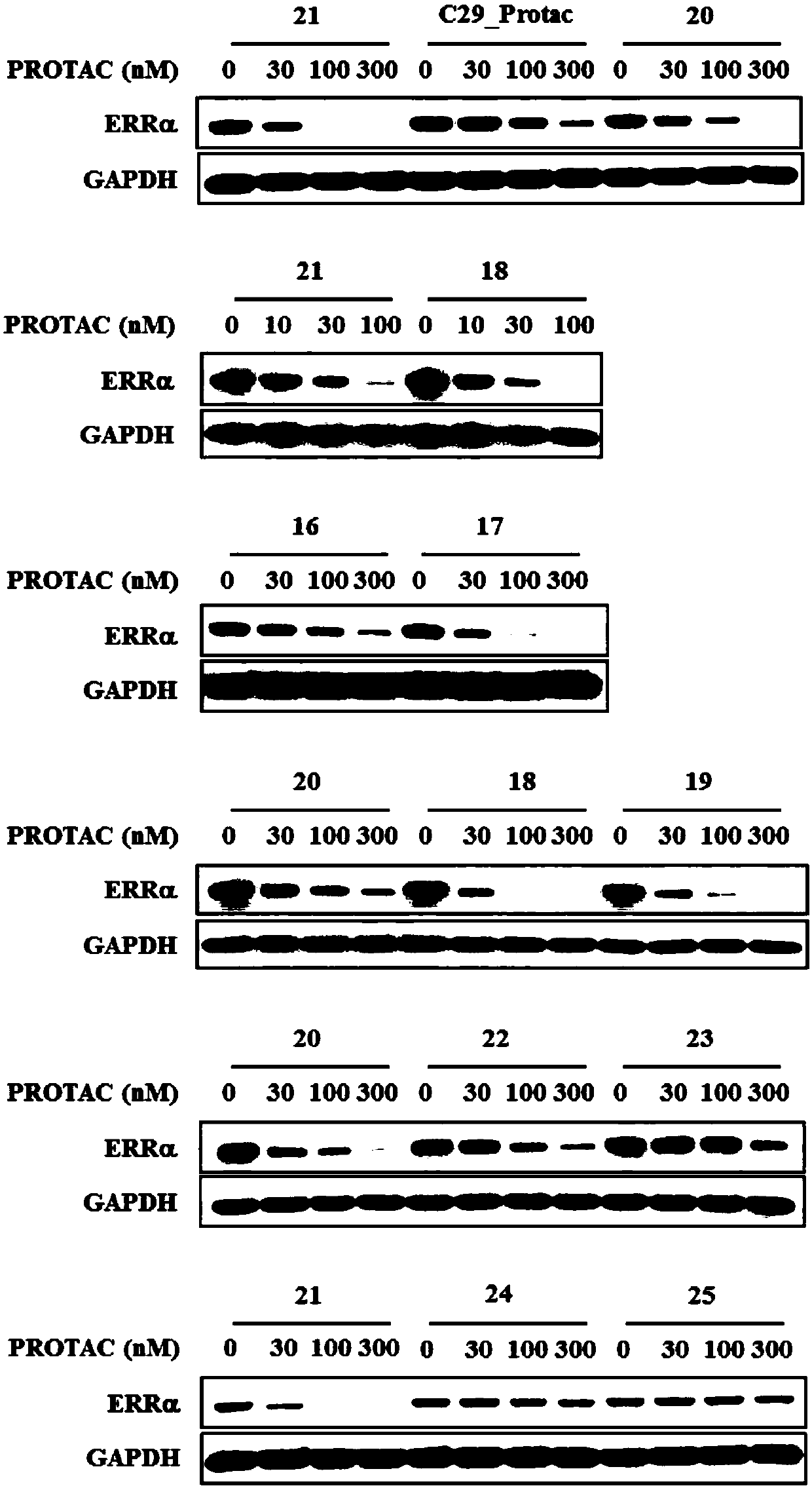
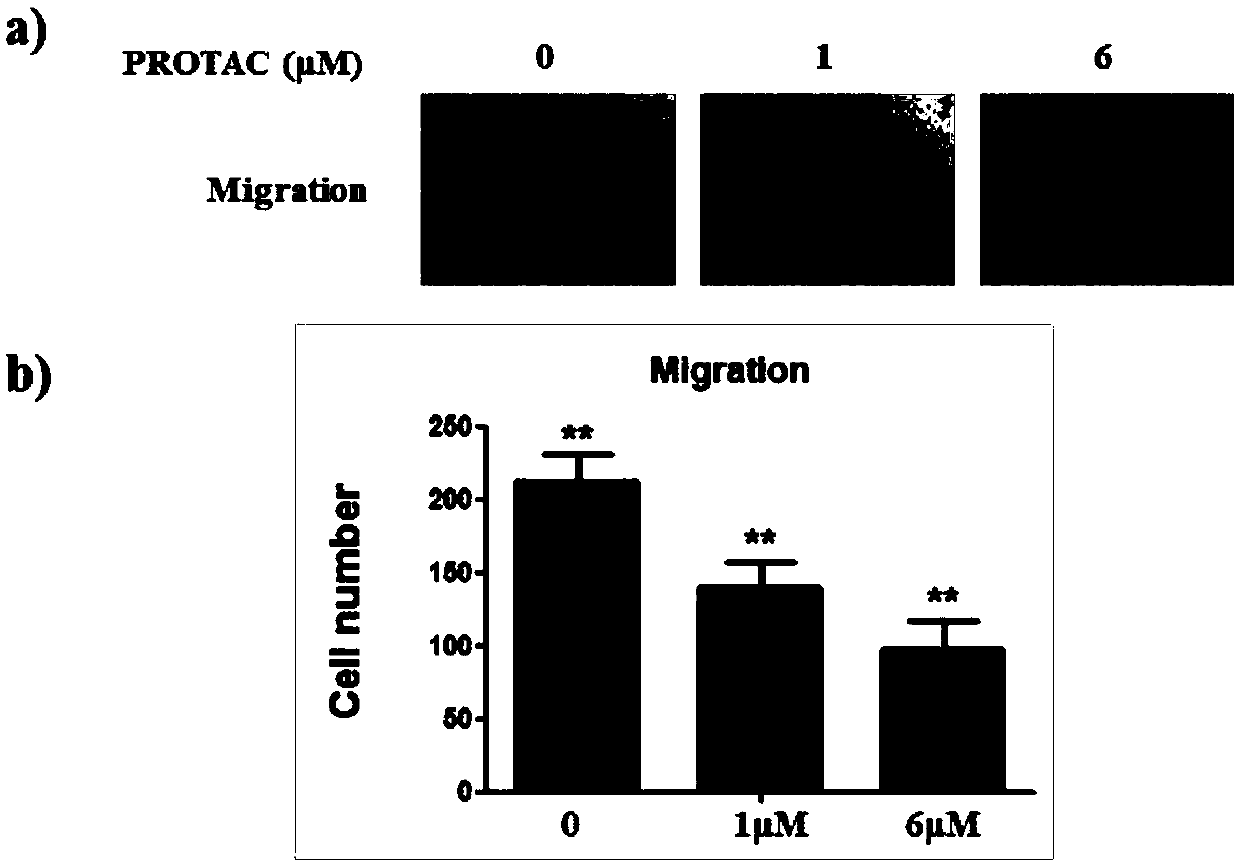
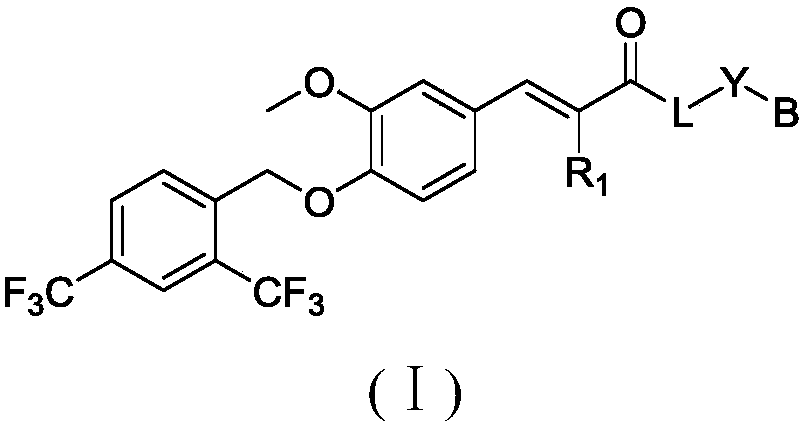
Compound for targeted ubiquitination degradation of ERRalpha protein and pharmaceutical composition and application thereof
A compound, alpha protein technology, applied in the direction of drug combinations, peptide/protein components, medical preparations containing active ingredients, etc., can solve the problems of limited development, large molecular weight, and drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1: (Z)-3-(4-((2,4-bis(trifluoromethyl)benzyl)oxy)-3-methoxyphenyl)-2-fluoroacrylic acid
[0168]
[0169] Step 1: Synthesis of 4-((2,4-bis(trifluoromethyl)benzyl)oxy)-3-methoxybenzaldehyde
[0170]
[0171] Dissolve compound 26 (3.07g, 10mmol) and vanillin (1.52g, 10mmol) in DMF (10ml), add K 2 CO 3 (2g, 14mmol), stirred at 80°C for 3 hours. After cooling to room temperature, the resulting mixture was extracted with ethyl acetate and water. The organic layer was separated, washed with brine, washed with Na 2 SO 4 dry. After the solvent was spin-dried under reduced pressure, the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=7:1) to obtain the product 27 (3.21 g, 8.50 mmol, 85%) as a white solid: 1 H NMR (400MHz, CDCl 3 )δ9.87(s,1H),7.97(d,J=8.4Hz,2H),7.85(d,J=8.5Hz,1H),7.48(d,J=1.7Hz,1H),7.42(dd, J=8.2,1.8Hz,1H),6.93(d,J=8.2Hz,1H),5.47(s,2H),3.99(s,3H).MS(ESI)m / z 379[M+H] + .
[0172] Step 2: Synthesis of ...
Embodiment 2
[0178] Example 2: (Z)-3-(4-((2,4-bis(trifluoromethyl)benzyl)oxy)-3-methoxyphenyl)-2-(trifluoromethyl) acrylic acid
[0179]
[0180] The difference from the steps in Example 1 is that after compound 27 is synthesized, the following reaction is carried out directly to prepare (Z)-3-(4-((2,4-bis(trifluoromethyl)benzyl)oxy)- 3-methoxyphenyl)-2-(trifluoromethyl)acrylic acid:
[0181] Compound 27 (3.78g, 10mmol), 3,3,3-trifluoropropionic acid (10mmol, 0.88ml) were dissolved in anhydrous THF (20ml) under argon protection. Titanium tetrachloride (16mmol, 1.8ml) was added dropwise at 0°C. Stirring was continued for 30 minutes and triethylamine (5.6ml, 40mmol) was added dropwise. After stirring overnight at room temperature, it was extracted with ethyl acetate and water. The organic layer was separated, washed with brine, washed with Na 2 SO 4 dry. After the solvent was spin-dried under reduced pressure, the residue was purified by silica gel column chromatography (petroleum ...
Embodiment 3
[0182] Example 3: (E)-3-(4-((2,4-bis(trifluoromethyl)benzyl)oxy-3-methoxyphenyl-2-cyanoacrylic acid
[0183]
[0184] The difference from the steps in Example 1 is that after compound 27 is synthesized, the following reaction is directly carried out:
[0185] Compound 27 (2.9g, 7.7mmol) and 2-cyanoacetic acid (977.9mg, 11.4mmol) were dissolved in acetonitrile (20ml), piperidine (1.5ml) was added, and stirred at 80°C for 3 hours. After cooling to room temperature, the resulting mixture was treated with water. Hydrochloric acid (2N) was then added to precipitate a solid. The mixture was extracted with ethyl acetate and water. The organic layer was separated, washed with brine, washed with Na 2 SO 4 dry. After the solvent was spin-dried under reduced pressure, the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=1:1) to obtain the product 3 as a pale yellow solid (2.6 g, 5.8 mmol, 75.6%): 1 H NMR (400MHz, DMSO-d 6 )δ13.79(s,1H),8.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com