Preparation method and application of hydroxyl cinnamyl ester type catechin
A technology of cinnamoyl ester type and catechin, applied in the field of natural medicinal chemistry, can solve the problems of inability to meet the reaction requirements, unable to complete the preparation, complex synthesis method and the like, and achieve the effects of easy implementation, low cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
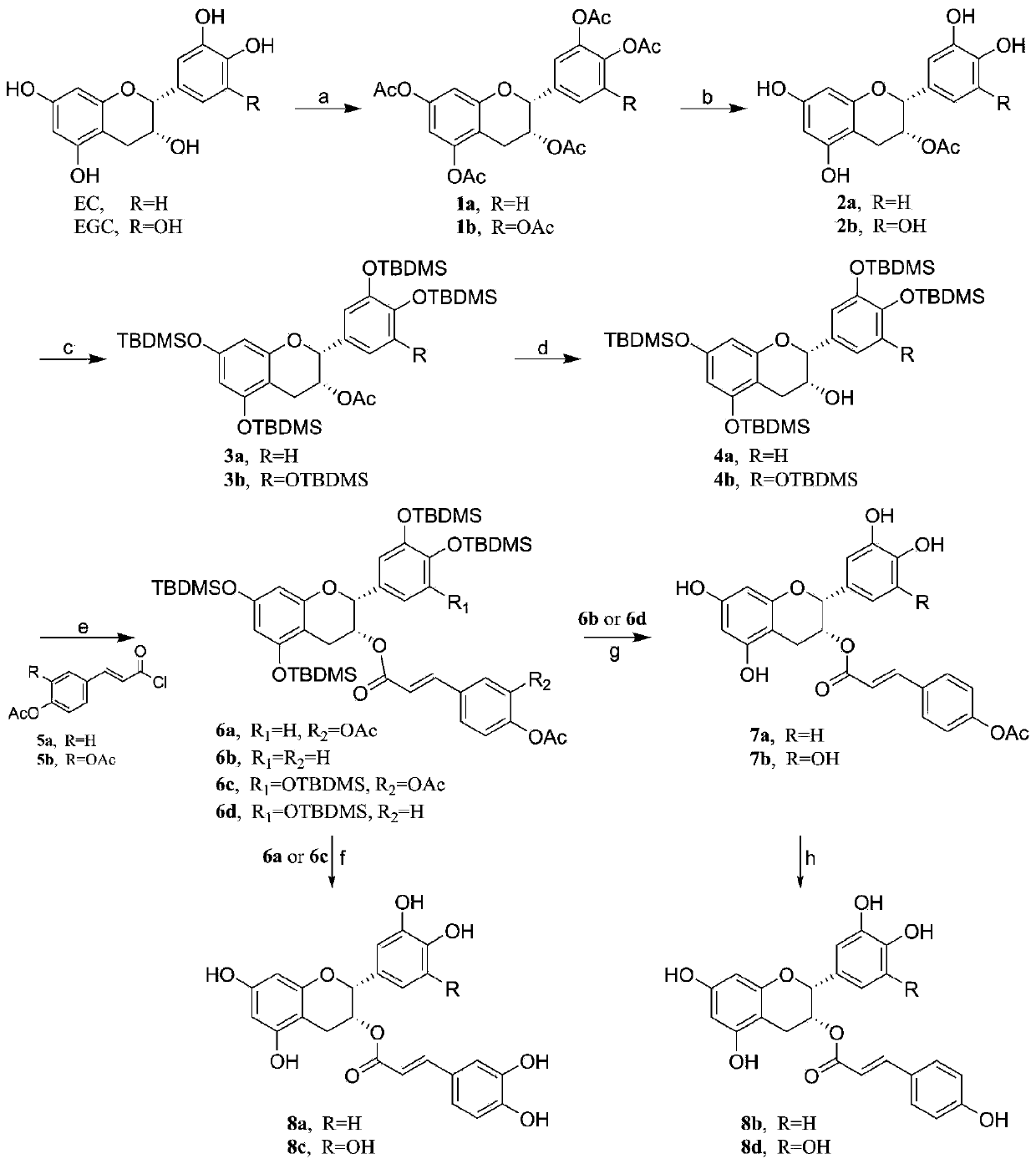
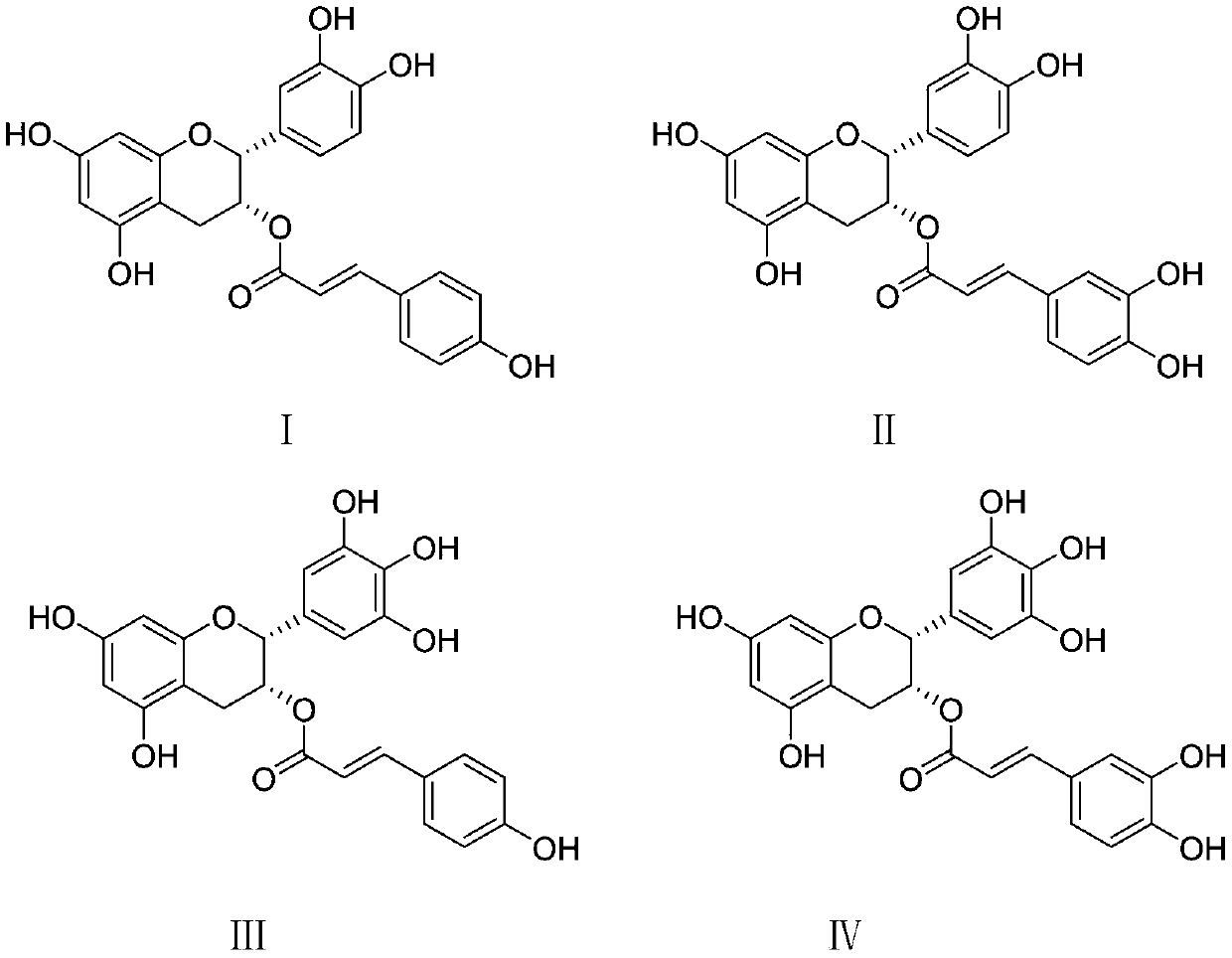
[0066] Epigallocatechin transcoumarate, epicatechin transcaffeate, epigallocatechin transcoumarate and epigallocatechin transcaffeate hydroxycinnamoyl ester type The preparation of tea;
[0067] 1.1 Instruments and reagents
[0068] 1 H nuclear magnetic resonance spectrum adopts Agilent DD2600 MHz nuclear magnetic resonance instrument; mass spectrum adopts Agilent6465UPLC-Q-TOF-MS.
[0069] Epicatechin (EC) and epigallocatechin (EGC) were purchased from Chengdu Biopharmaceutical Co., Ltd. and Hubei Jusheng Technology Co., Ltd.; caffeic acid and p-coumaric acid were purchased from Anaiji Chemical; other reagents were Domestic analytically pure.
[0070] 1.2 Epicatechin trans-coumarate, epicatechin trans-caffeate, epigallocatechin trans-coumarate and epicatechin trans-caffeate hydroxycinnamoyl esters The synthesis route of tea is as follows figure 1 shown;
[0071] 1.3 Specific implementation methods:
[0072] Synthesis of compound 1a:
[0073] Weigh 1.16g (4.0mmol) of e...
Embodiment 2
[0113]The epicatechin trans-coumarate, epicatechin trans-caffeate, epigallocatechin trans-coumarate and epigallocatechin trans-caffeate prepared in Example 1 Four hydroxycinnamoyl catechins were tested for their inhibition of α-glucosidase activity in vitro.
[0114] 2.1 Experimental materials and reagents
[0115] Acarbose, potassium phosphate buffer (PPBS, 10mM, pH 6.9), α-glucosidase (0.1U·mL -1 ), 4-nitrophenyl-α-D-glucopyranoside (pNPG, 2.5mM), dimethylsulfoxide (DMSO)
[0116] 2.2 Experimental methods and results
[0117] Add 60 μL of α-glucosidase solution to a 96-well plate, add 60 μL of four hydroxycinnamoyl ester catechins with different concentration gradients, and pre-incubate at 37°C for 10 minutes, add 60 μL of pNPG solution respectively, and continue to After incubating at 37°C for 20 minutes, place it in a microplate reader (25°C), and record the A value of the reaction solution under visible light with a wavelength of 405nm. All samples were repeated 3 tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com