Method for semi-continuously synthesizing trimethyl borate-methanol azeotrope
A trimethyl borate, semi-continuous technology, applied in the chemical industry, can solve the problems of low conversion rate and difficult water discharge, and achieve the effect of stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
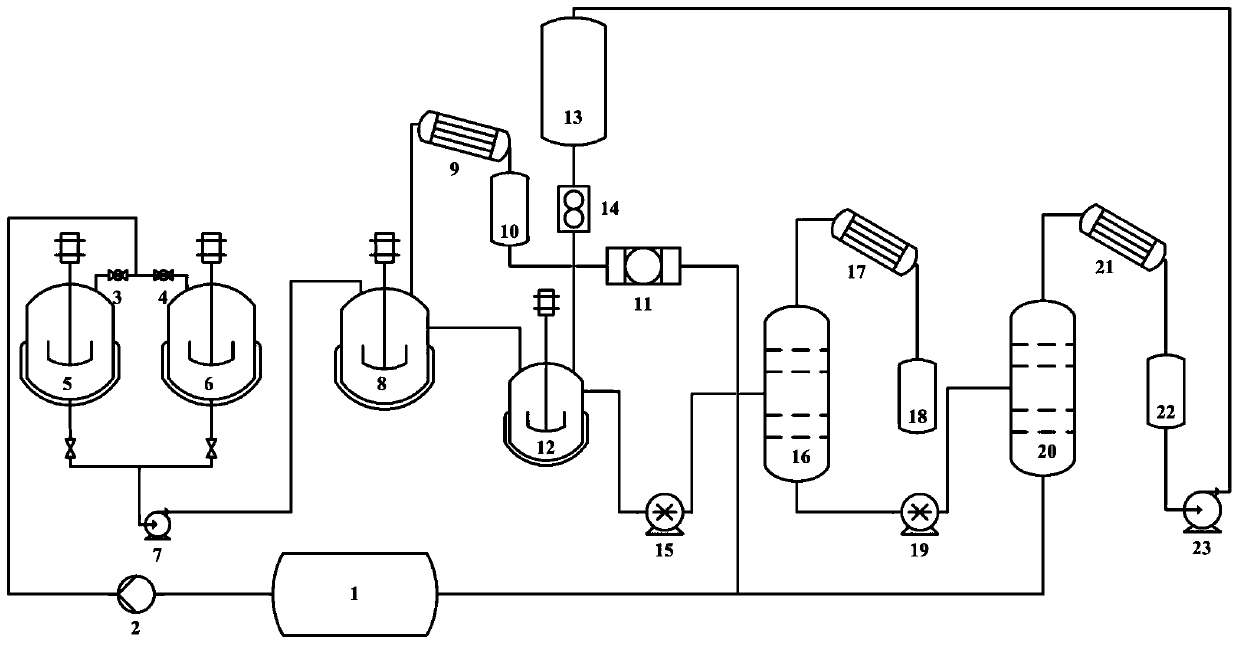
[0023] Butanol (C 4 h 10 0) Extracted by the first storage tank 1 through the feed pump 2, the first valve 3 is opened, the second valve 4 is closed, n-butanol is introduced into the first premix tank 5 with a volume of 25L, and the After adding 15L of n-butanol in 5, close the first valve 3, start stirring, add 1.67kg boric acid to the first premix tank 5 at the same time, stir and heat to 85°C, stir and keep warm for 0.5h, open the bottom valve and pass through the first inlet The feed pump 7 continuously imports the mixed solution into the continuous stirring and heating reactor 8 (volume is 30L), and the control flow rate is 15L / h; after opening the valve at the bottom of the first premixing tank 5, open the second valve 4, and the positive Butanol is introduced into the second premixing tank 6 for a new round of boric acid and n-butanol mixing process; the temperature of the mixed solution rises to 130°C after entering the reactor 8, and the generated gas enters the firs...
Embodiment 2
[0025] Triethanolamine (C 6 h 15 NO 3) is extracted from the first storage tank 1 through the feed pump 2, the first valve 3 is opened, the second valve 4 is closed, and triethanolamine is introduced into the first premix tank 5 with a volume of 25L, and the first premix tank 5 is added After 15L of triethanolamine, close the first valve 3 and start stirring. At the same time, add 3.5kg of boric acid to the first premix tank 5, stir and heat to 90°C, stir and keep warm for 1 hour, open the bottom valve and pass the first feed pump 7 to mix The liquid is continuously introduced into the continuously stirring and heated reactor 8 (volume is 30L), and the control flow rate is 5L / h; after opening the valve at the bottom of the first premixing tank 5, open the second valve 4, and triethanolamine is imported into the second premixing tank. A new round of boric acid and triethanolamine mixing process is carried out in the mixing tank 6; the temperature of the mixed solution rises t...
Embodiment 3
[0027] ethanolamine (C 2 h 7 NO) is extracted from the first storage tank 1 through the feed pump 2, the first valve 3 is opened, the second valve 4 is closed, the ethanolamine is introduced into the first premix tank 5 with a volume of 25 L, and the first premix tank 5 is added After 15L of ethanolamine, close the first valve 3, start stirring, and add 4.2kg of boric acid to the first premix tank 5 at the same time, stir and heat to 90°C, stir and keep warm for 1 hour, open the bottom valve and pass the mixed solution through the first feed pump 7 Continuously import in the reactor 8 (capacity is 30L) of continuous stirring and heating, control flow rate is 10L / h; After opening the valve at the bottom of the first premix tank 5, open the second valve 4, ethanolamine is imported into the second premix tank In 6, a new round of boric acid and ethanolamine mixing process is carried out; after the mixed solution enters the reaction kettle 8, the temperature rises to 180°C, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com