Method for simultaneously determining concentrations of ticagrelor and active metabolites and endogenous adenosine thereof in human plasma by liquid chromatography-mass spectrometry
A technology of ticagrelor and liquid chromatography-mass spectrometry, applied in the field of medical testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Pre-experiment
[0048] 1. Experimental materials
[0049] 30% perchloric acid, 30% acetonitrile, 50% acetonitrile, 80% acetonitrile, 100% acetonitrile, ticagrelor, AR-C124910XX
[0050] 2. Experimental method
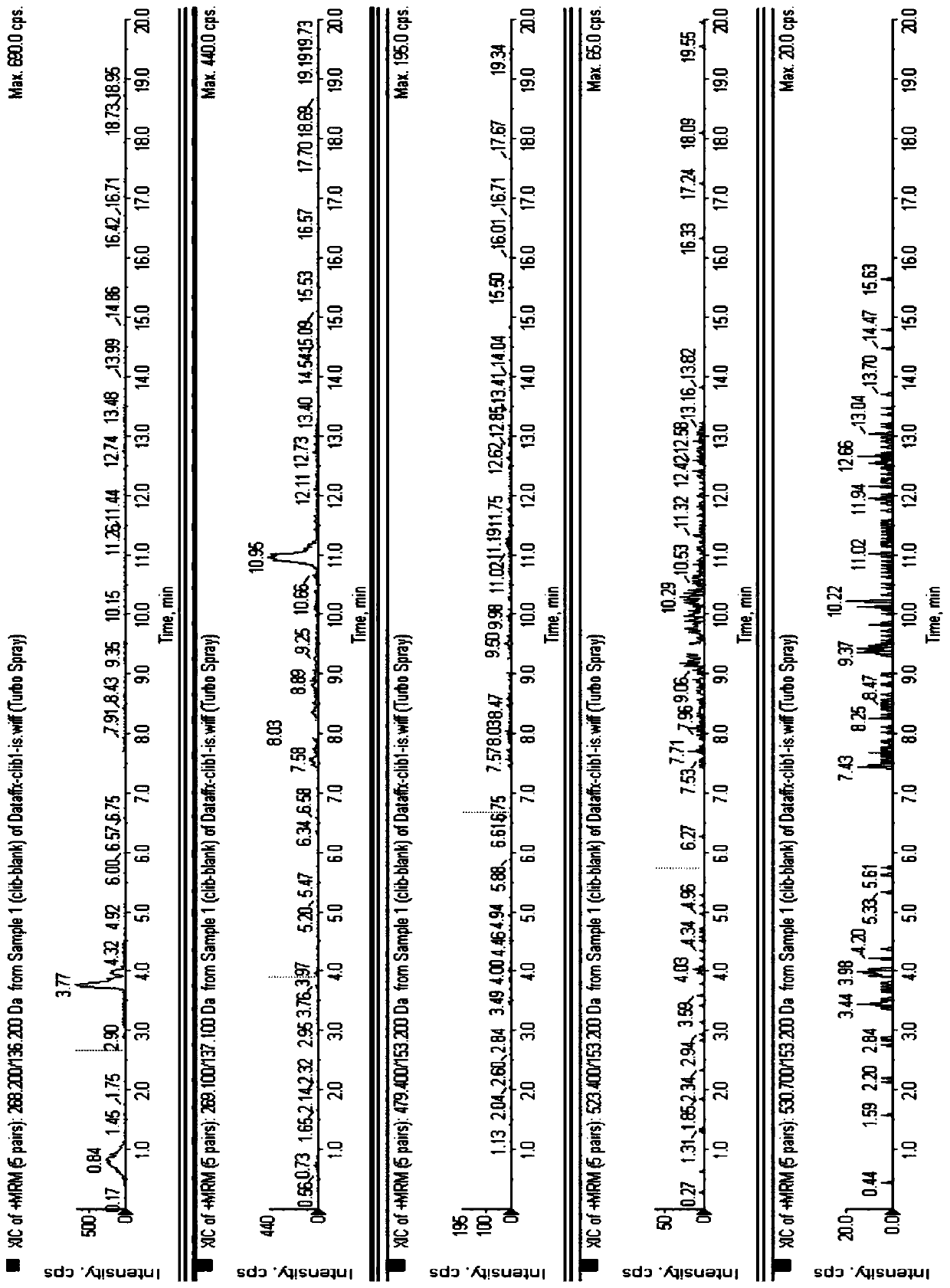
[0051] Adenosine in the sample to be tested is unstable, so the present invention selects 30% perchloric acid to precipitate protein, effectively removes enzymes in biological samples, and ensures that the concentration of adenosine is stable after pretreatment.
[0052] 3. Experimental results
[0053] In the present invention, adenosine to be tested is a very polar compound, and ticagrelor and its active metabolites are very weak in polarity. Therefore, the present invention investigates the ratio of organic solvents in different proportions. After dissolving perchloric acid, its extraction and recovery The rate difference can be seen in Table 1.
[0054] Table 1, different proportions of acetonitrile-aqueous solution dissolved perchloric acid...
Embodiment 2
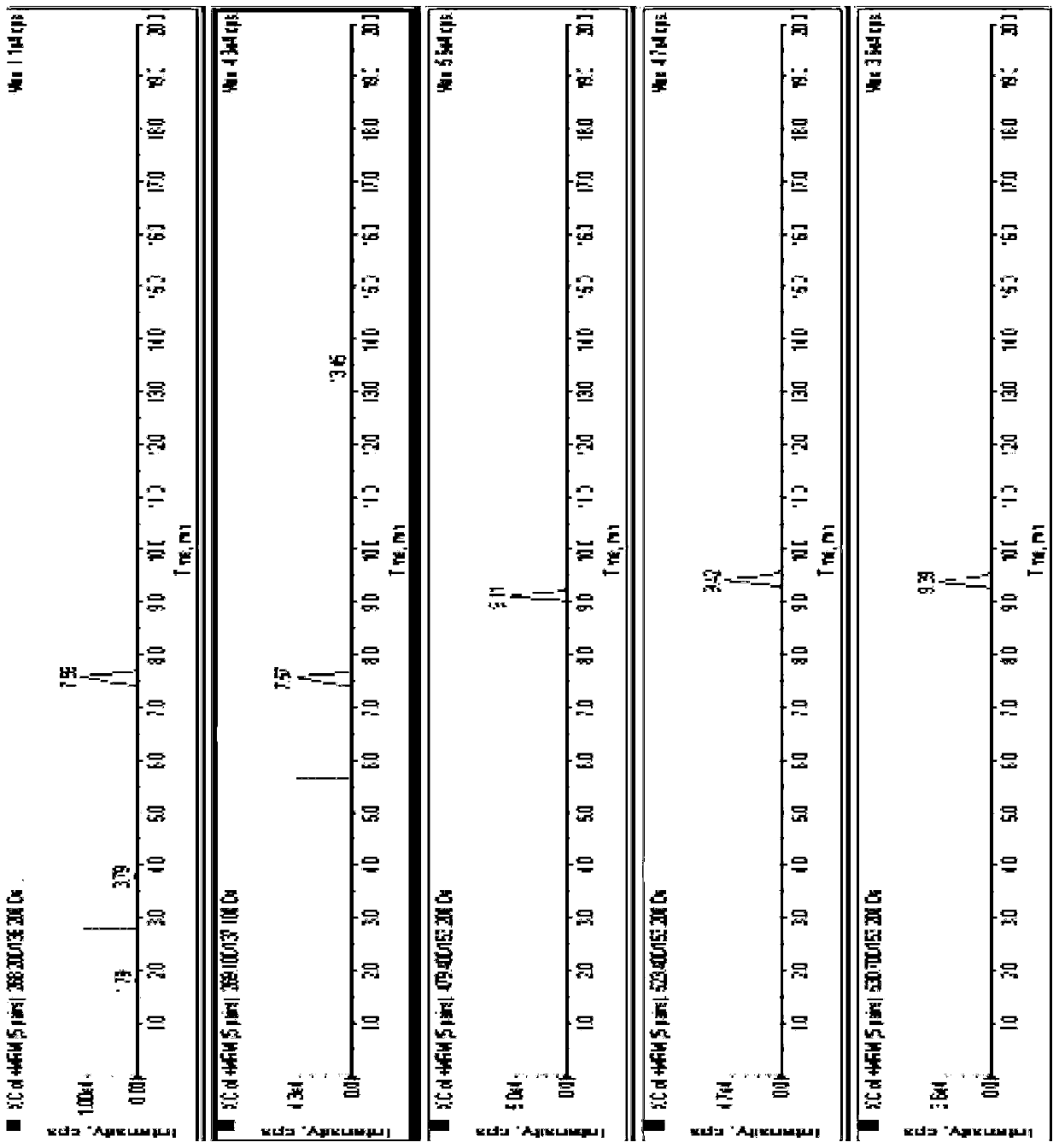
[0058] Embodiment 2 Selectivity and linearity test
[0059] 1. Experimental method
[0060] Biological blank matrix treatment: put the plasma sample for a period of time until the adenosine disappears completely (2 hours after the adenosine disappears completely), add 50μl stabilizer to 1ml of plasma, mix the plasma and set aside.
[0061] Linearity test:
[0062] Configure a series of standard curve plasma samples, including adenosine concentrations of 110, 55, 11, 5.5, 3.3, 2.2ng / mL; ticagrelor 1600, 800, 160, 80, 48, 32ng / mL; ticagrelor activity Metabolites 1108, 554, 110.8, 55.4, 33.2, 22.2 ng / mL. According to the plasma sample pretreatment operation. Taking the concentration of the analyte (C, ng / mL) as the abscissa, and the ratio (As / Ai) of the peak area of the analyte (As) to the internal standard peak area (Ai) as the ordinate, use weighting (weighting factor: 1 / C 2 ) least squares method for linear regression, regression equation correlation coefficient (r) gr...
Embodiment 3
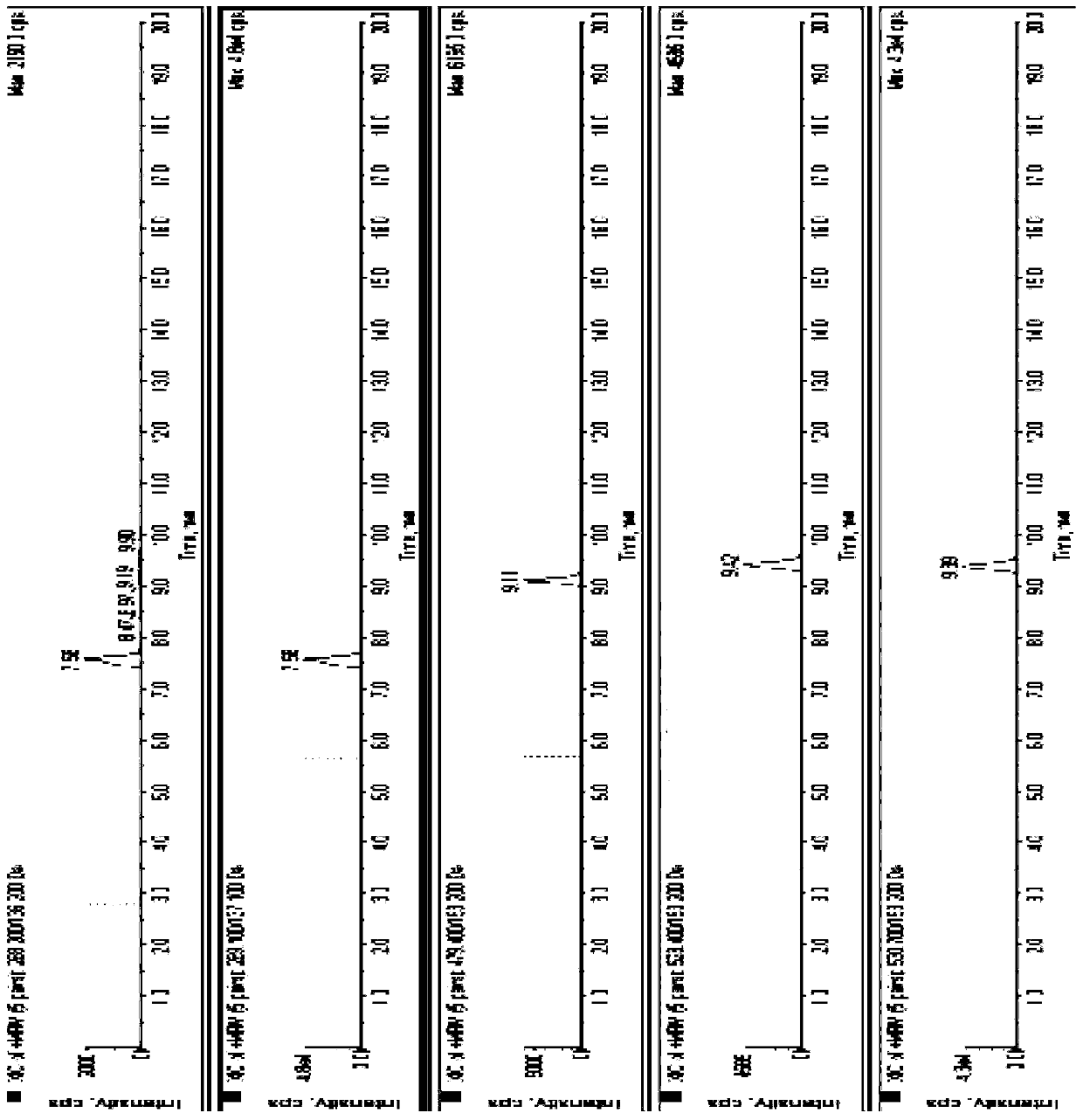
[0069] Embodiment 3 extracts recovery rate and matrix effect
[0070] 1. Experimental method
[0071] Take the blank plasma treated with the blank matrix, except that the standard series solution and the internal standard solution are not added, and the supernatant is prepared according to the "plasma sample pretreatment method" in Example 2. Add the corresponding concentration standard series solution and internal standard to the supernatant, analyze 5 samples for each concentration, and obtain the corresponding peak area value A. Take 200 μL of blank plasma treated with blank matrix, prepare standard series solutions and internal standard solutions of corresponding concentrations, operate according to the "plasma sample pretreatment method" in Example 2, analyze 5 samples for each concentration, and obtain the corresponding peak area value B. In addition to preparing standard series solutions and internal standard solutions of corresponding concentrations, analyze 5 samples...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com