The preparation method of dl-p-thymphenyl phenylserine ester
A technology of methylsulfonyl phenylserine ethyl ester and methanol, which is applied in the field of preparation of DL-p-thymphenyl phenylserine ester, can solve the problems of long recycling period and low production capacity, so as to improve efficiency, simplify production process, reduce pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
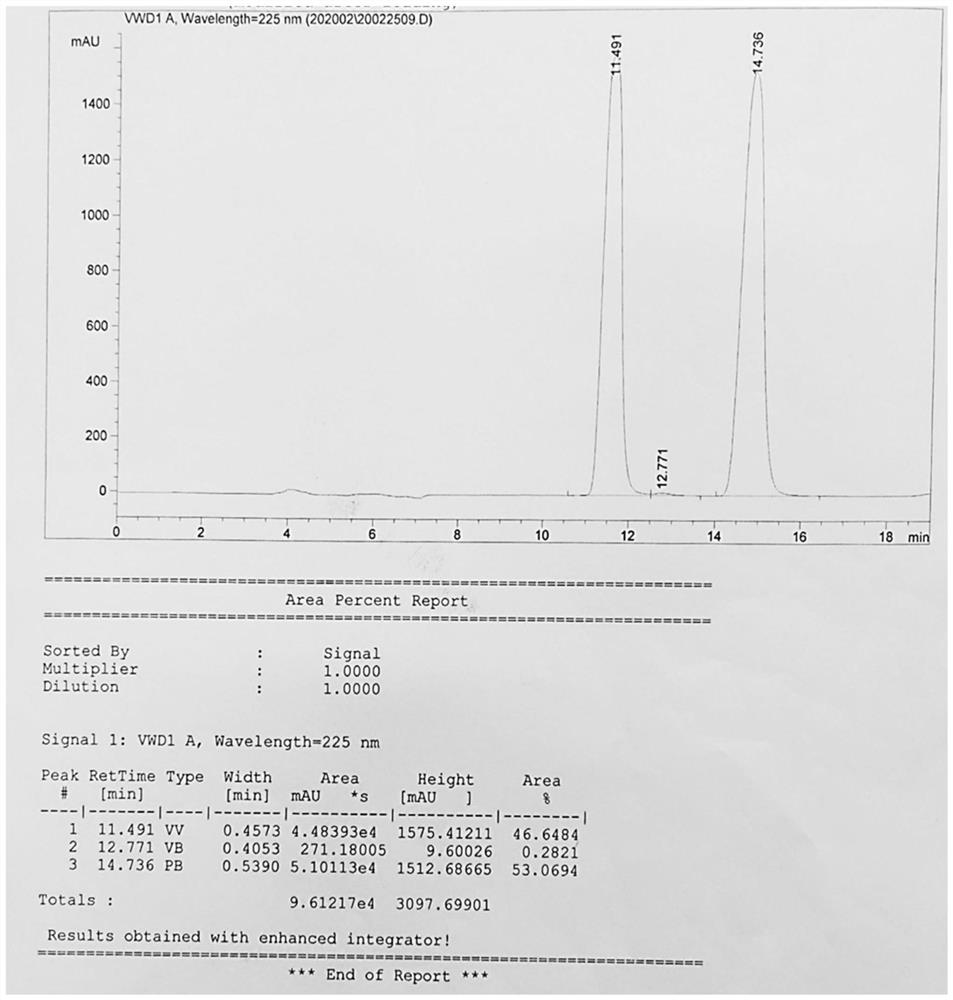
[0038] Take L- p-methyl sulfonylphenylserine ethyl ester 28.7g (0.1mol), disperse it into 170g of water, control the temperature at 15 °C (temperature difference ± 2 °C), insulation stirring for 30 minutes, add a concentration of 30wt% sodium hydroxide solution 3.9g, insulation reaction for 8 hours, after which the pH value was adjusted to 7.0, and then filtered to obtain the product D- p-methyl sulfonyl phenylserine ethyl ester and L- p-methylsulfonylphenylserine ethyl ester for a total of 18.52g. as Figure 1 As shown, the characteristic peak at 11.491min is the characteristic peak of D- p-methylsulfonyl phenylserine ethyl ester, and the characteristic peak at 14.736min is L- p-methylsulfonylphenylserine ethyl characteristic peak, and the ratio of D- p-methylsulfonylphenylserine ethyl ester and L- p-methylsulfonylphenylserine ethyl ester is 46.6%:53.1%, that is, 1:1.1, close to 1:1.
Embodiment 2
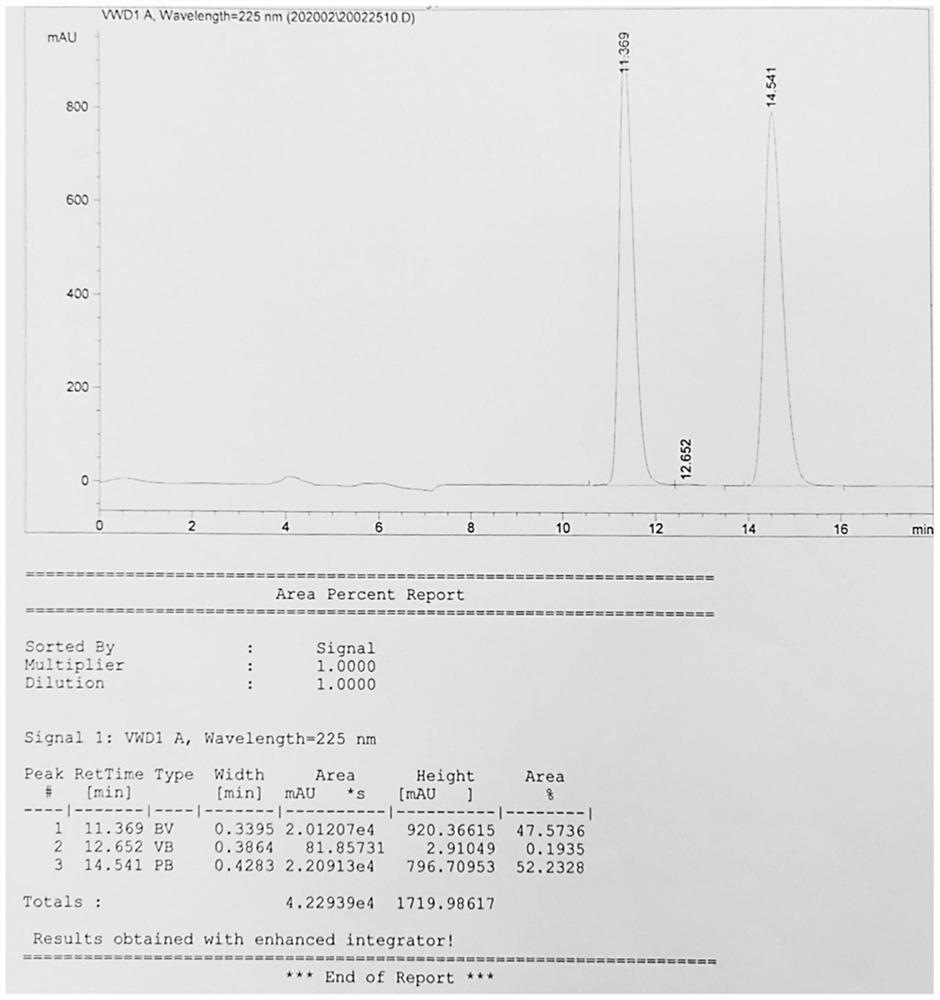
[0040] Take L- p-methyl sulfonylphenylserine ethyl ester 28.7 g (0.1 mol), dispersed into 170 g ethanol, the temperature was controlled at 15 °C (temperature difference ± 2 °C), after heat preservation stirring for 30 minutes, the concentration of 10% sodium hydroxide solution 11.5 g, the insulation reaction for 10 hours, and then the pH value was adjusted to 7.0, filtered to give the product D- p-methyl sulfone phenyl serine ethyl ester and L- p-methyl sulfone phenyl serine ethyl ester a total of 15.32 g. as Figure 2 As shown, at 11.369min the characteristic peak is the characteristic peak of D- p-methylsulfonylphenylserine ethyl ester, at 14.541min the characteristic peak is L- p-methylsulfonylphenylserine ethyl ester characteristic peak, the ratio of D- p-methyl sulfonylphenylserine ethyl ester and L- p-methylsulfonylphenylserine ethyl ester is 47.6%: 52.2%, that is, 1: 1.1, close to 1: 1.
Embodiment 3
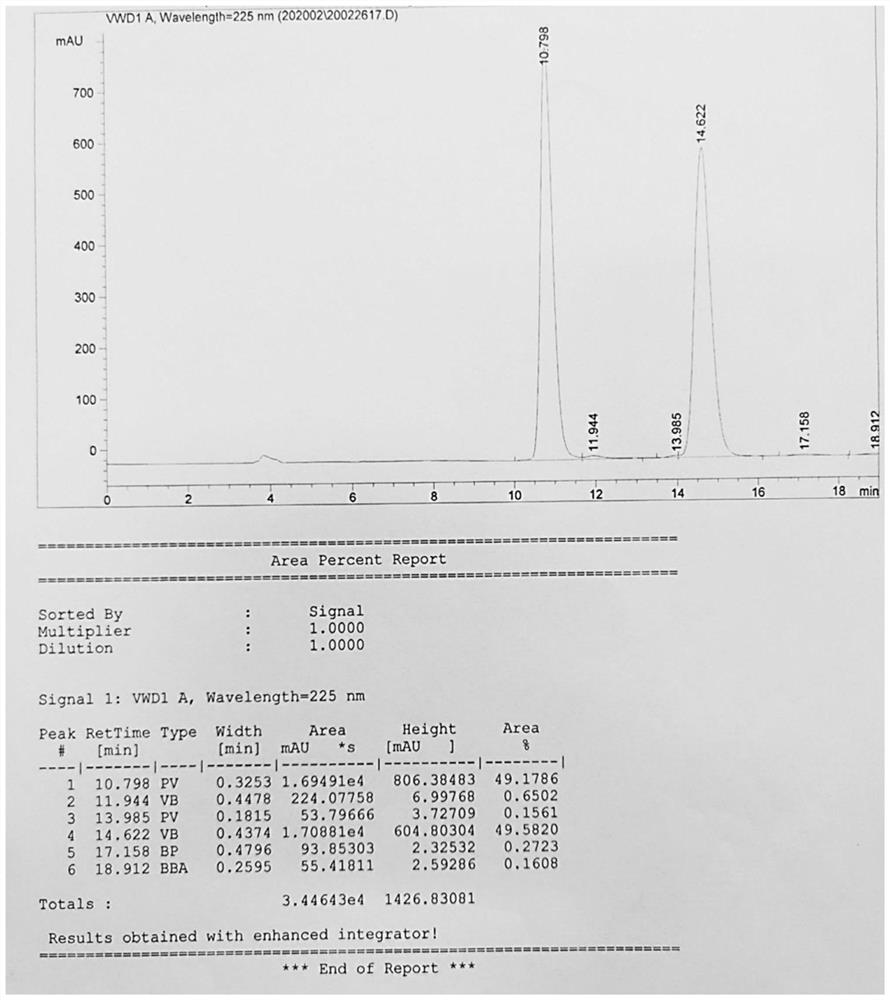
[0042] Take L- p-methyl sulfonylphenylserine ethyl ester 28.7g (0.1mol), dispersed into 170g of methanol, the temperature was controlled at 15 °C (temperature difference ±2 °C), stirred for 30 minutes, with 10% sodium hydroxide solution 11.5g, insulation reaction for 10 hours, and then the pH value was adjusted to 7.0, filtered to give the product D- p-methylsulfonyl phenylserine ethyl ester and L- p-methyl sulfonylphenylserine ethyl ester a total of 13.32g. as Figure 3As shown, at 10.798min the characteristic peak is D- p-methylsulfonylphenylserine ethyl characteristic peak, at 14.622min the characteristic peak is L- p-methylsulfonylphenylserine ethyl characteristic peak, the ratio of D- p-methylsulfonylphenylserine ethyl ester and L- p-methylsulfonylphenylserine ethyl ester is 49.2%: 49.6%, that is, 1: 1.0, close to 1: 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com