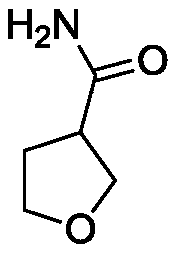
Synthesis method for 3-aminomethyl tetrahydrofuran
A technology of aminomethyltetrahydrofuran and cyanotetrahydrofuran, which is applied in the field of synthesis of 3-aminomethyltetrahydrofuran, can solve problems such as high technical requirements, unfavorable industrialization, and harsh conditions, and achieve simple process, green method, and industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
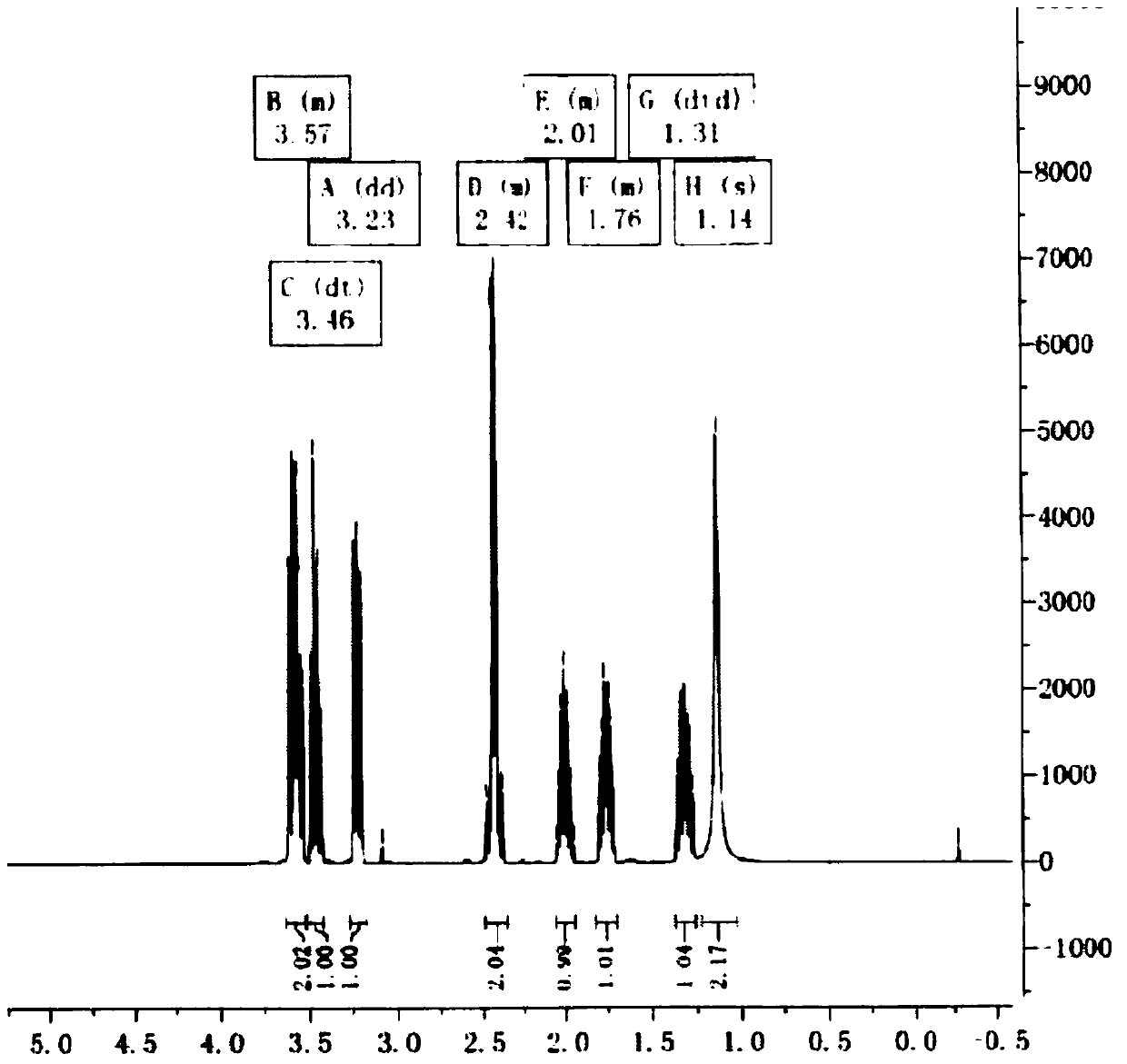
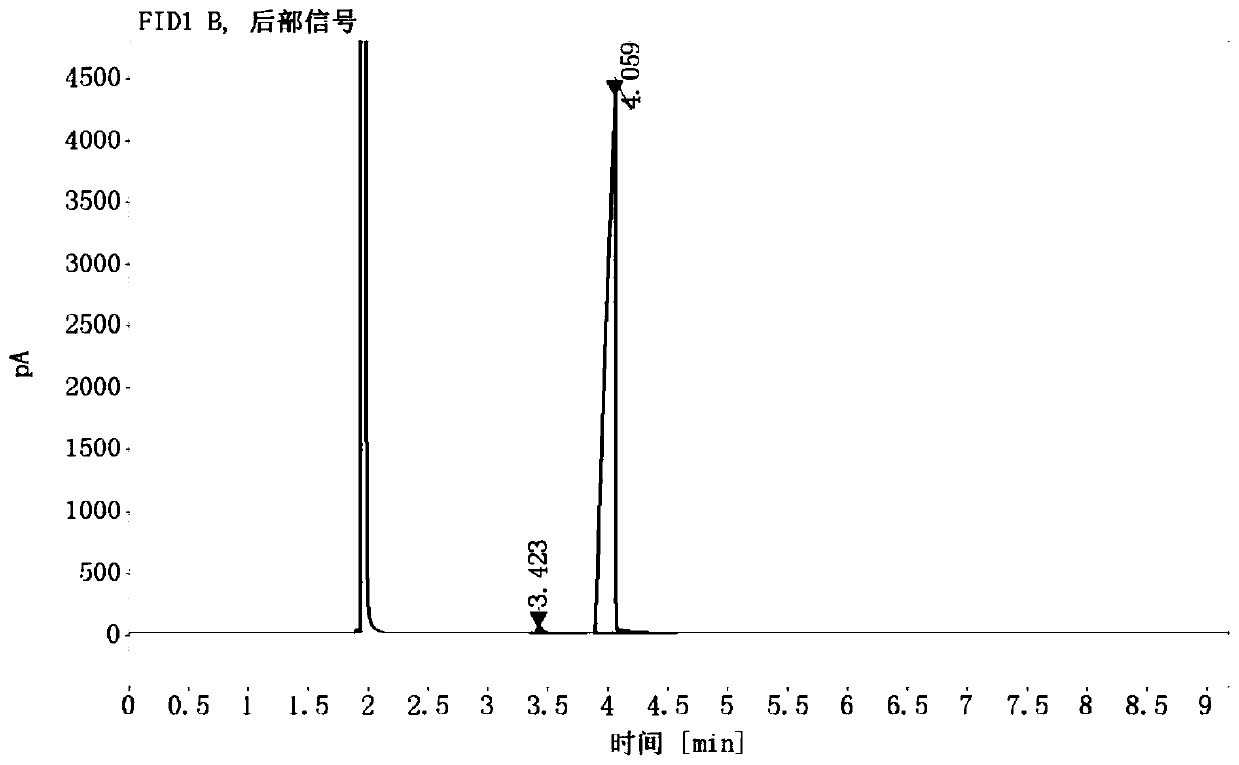
Image
Examples
Embodiment 1
[0055] A kind of synthetic method of 3-aminomethyltetrahydrofuran, specifically comprises the steps:
[0056](1) Ammonium chloride (5.35g, 0.1mol), 2,5-dihydrofuran (7.01g, 0.1mol), palladium dichloride (0.0177g, 0.0001mol) were added to 20mL of N-methylpyrrolidone Then put it into a high-pressure reactor, replace the air in the reactor with carbon monoxide, fill it with carbon monoxide (0.1MPa) after three replacements, stop the reaction after reacting in an oil bath at 200°C for 24 hours, evaporate the solvent to dryness, and weigh it with ethanol Crystallization obtained 7.63 g of an off-white solid, namely 3-formamide tetrahydrofuran, with a purity of 95% and a yield of 63%;
[0057] (2) Dissolve the off-white solid (6.06g, 0.05mol of 3-formamide tetrahydrofuran) obtained in step (1) in toluene (50mL), then add phosphorus pentachloride (10.41g, 0.05mol), and Stir for 20 minutes, then heat up to 50°C and stir for 30 minutes. After the reaction is complete, wash with 10 mL ...
Embodiment 2
[0062] A kind of synthetic method of 3-aminomethyltetrahydrofuran, specifically comprises the steps:
[0063] (1) Add ammonium chloride (107g, 2mol), 2,5-dihydrofuran (14.02g, 0.2mol), palladium acetate (4.49g, 0.02mol) into 40mL of tetrahydrofuran, and then put it into the autoclave In the process, replace the air in the reactor with carbon monoxide, and after three replacements, fill it with carbon monoxide (10MPa), react in an oil bath at 50°C for 24 hours, then stop the reaction, evaporate the reaction solution to dryness, and recrystallize with ethanol to obtain off-white 15.73 g of solid, namely 3-formamide tetrahydrofuran, with a purity of 95% and a yield of 65%;
[0064] (2) The off-white solid (6.06g, 0.05mol of 3-formamide tetrahydrofuran) obtained in step (1) was dissolved in diphenyl ether (50mL), and then dibutyltin oxide (1.245g, 0.005mol) was added, Heat to 200°C for 1.5h until no water is distilled off, filter while hot to remove dibutyltin oxide, concentrate ...
Embodiment 3
[0067] A kind of synthetic method of 3-aminomethyltetrahydrofuran, specifically comprises the steps:
[0068] (1) Ammonium bromide (48.98g, 0.5mol), 2,5-dihydrofuran (14.02g, 0.2mol), sodium chloropalladate (2.942g, 0.01mol) were added to 40mL of N,N-dihydrofuran Methylformamide, then put it into a high-pressure reactor, replace the air in the reactor with carbon monoxide, after three replacements, fill it with carbon monoxide (3MPa), react in an oil bath at 100°C for 24 hours, stop the reaction, and distill under reduced pressure , collect the required fractions and recrystallize with ethanol after cooling to obtain 15.75 g of off-white solid, which is 3-formamide tetrahydrofuran, with a purity of 95% and a yield of 65%;
[0069] (2) Dissolve the off-white solid (4.24g, 0.035mol of 3-formamide tetrahydrofuran) obtained in step (1) in toluene (50mL), then add phosphorus oxychloride (9.79mL, 0.105mol) and di Aluminum (0.036g, 0.00035mol) was heated to reflux for 2h until no wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com