Recombinant Escherichia coli producing n-acetyl-5-hydroxytryptamine and its construction method and application
A technology for recombining Escherichia coli and Escherichia coli, which is applied in the field of genetic engineering, can solve problems such as low protein specific activity, low expression of inclusion bodies, and expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
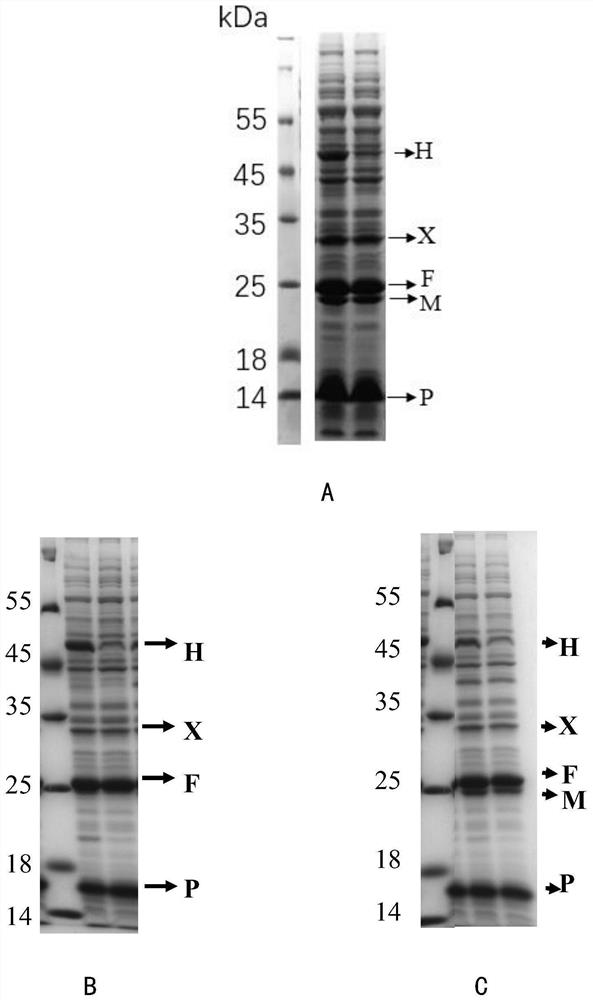
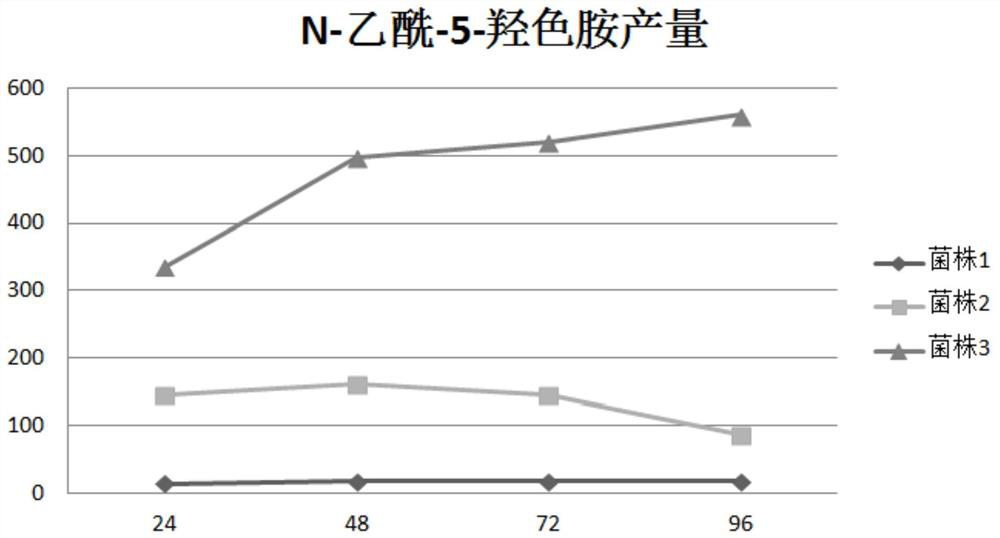
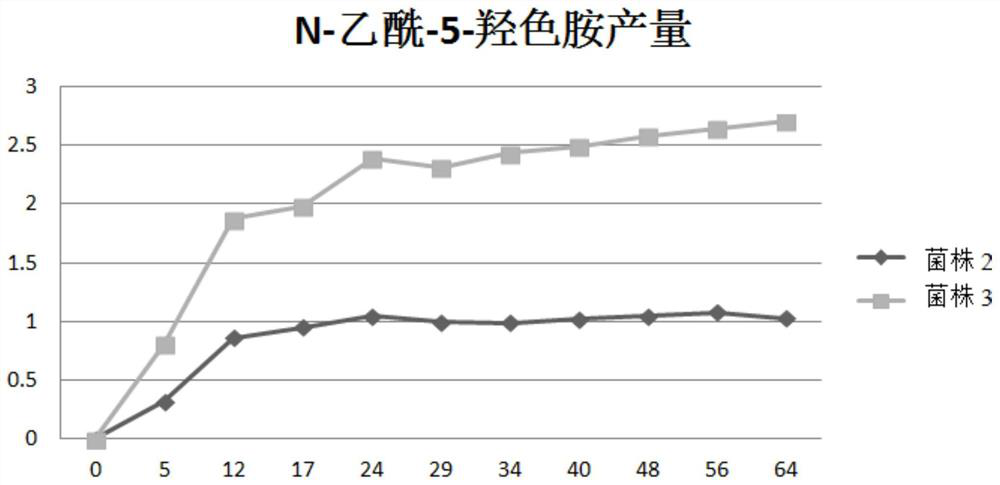
[0125] Embodiment 1, the construction of the recombinant Escherichia coli strain with high yield of N-acetyl-5-hydroxytryptamine
[0126] 1. PCR amplification of the coding sequence of proteins related to the synthesis of tryptophan derivatives
[0127] Using the synthetic phenylalanine hydroxylase gene (shown in positions 319-1209 of SEQ ID NO.1) derived from Xanthomonas campestris as a template, PCR amplification was performed with primers F1 and R1 to obtain a phenylalanine hydroxylase gene containing benzene PCR amplification product X of alanine hydroxylase gene (represented by positions 299-1232 of SEQ ID NO.1).
[0128] Using the synthetic 4a-hydroxytetrahydrobiopterin dehydratase gene phhB (shown in 1234-1590 of SEQ ID NO.1) derived from Pseudomonas aeruginosa as a template, PCR amplification was performed with primers F3 and R3 to obtain PCR amplification product P of 4a-hydroxytetrahydrobiopterin dehydratase gene phhB (represented by positions 1210-1614 of SEQ ID NO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com