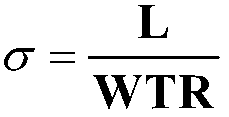Ionic liquid-based composite alkaline electrolyte membrane with electrostatic interaction, and preparation and application thereof
An ionic liquid, electrolyte membrane technology, applied in circuits, fuel cells, electrical components, etc., can solve problems such as the degradation of alkaline membrane performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
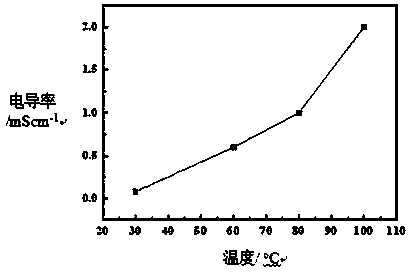
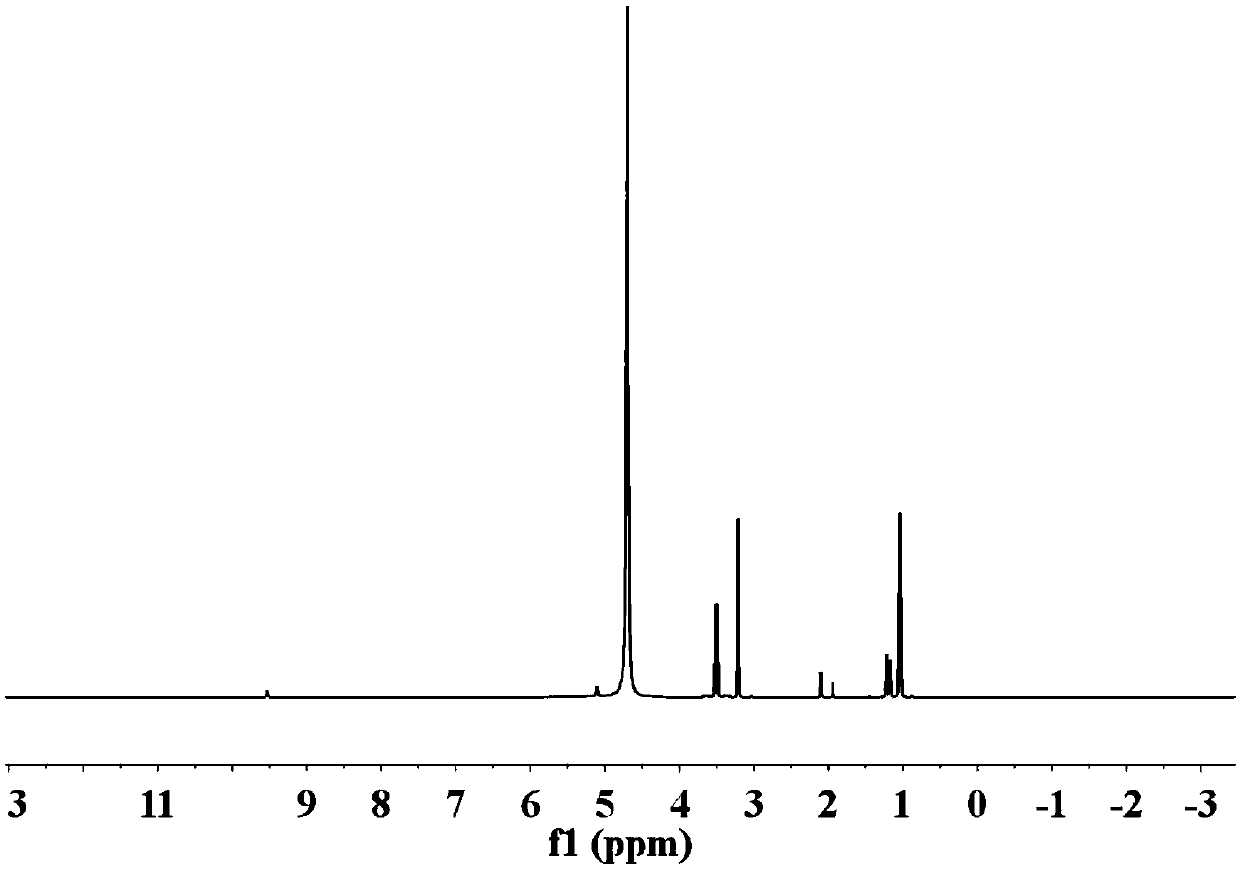
Image
Examples
Embodiment 1
[0047] Add 20g of N-methylimidazole and 40mL of bromoethane into a 100mL single-necked round bottom flask, and react at room temperature for 6h to obtain 1-methyl-3-ethylimidazolium bromide. Dissolve 100g LiTFSI in 1L deionized water, add the 1-methyl-3-ethylimidazolium bromide prepared above into the LiTFSI / water solution, stir thoroughly at room temperature for 36h, separate the liquids, and then use 50g LiTFSI / 1L The aqueous solution was treated once, and after liquid separation, it was dried in a vacuum oven at 120°C for 48 hours, and vacuum was drawn once every 4 hours during drying.
[0048] Dissolve 5 g of the above-prepared 1-methyl-3-ethylimidazole TFSI type ionic liquid in 1 mL of ethanol, and add it to 5 mL to a concentration of 0.2 g mL -1 In the Nafion / ethanol solution, the gel-like substance was obtained, filtered, dried at 40°C for 30min under vacuum, placed in a PTFE film, placed between two steel plates, and hot-pressed at 5000Pounds and 100°C for 1h to obtain...
Embodiment 2
[0056] Add 25g of methylpiperidine and 30mL of bromoethane into a 100mL single-necked round bottom flask, and react at 70°C for 2h to obtain N-methyl-ethylpiperidine bromide. Dissolve 10g of N-methyl-ethylpiperidine bromide prepared in 10mL of water, add 50g LiTFSI / 1L deionized aqueous solution to it, stir thoroughly for 36h, separate the liquids, and then treat with 20g LiTFSI / 300mL LiTFSI / water solution 28h, after liquid separation, dry in a vacuum oven at 120°C for 48h.
[0057] The N-methyl-ethylpiperidine TFSI-type ionic liquid prepared above was dissolved in different volumes of ethanol to obtain four solutions with concentrations of 1:10, 1:8, 1:3, and 1:0 g / mL. The Nafion membrane with short side chains was placed in the above four parts of ionic liquid / ethanol solution in turn for 4 hours, and the prepared alkaline electrolyte membrane with ionic liquid with electrostatic effect was hot-pressed at 1000 Pounds for 0.5 h at 100°C to finally obtain electrostatic A relat...
Embodiment 3
[0059] Add 25g of methylpiperidine and 30mL of bromoethane into a 100mL single-necked round bottom flask, and react at 70°C for 2h to obtain N-methyl-ethylpiperidine bromide. Dissolve 10 g of N-methyl-ethylpiperidinium bromide prepared in 15 mL of acetone, add 20 g of NaBF 4 / 250mL acetone solution, stirred for 36h, and then used the same method in 20g NaBF 4 / 250mL acetone solution for 4 times, the liquid was filtered and evaporated to remove acetone, and finally dried in a vacuum oven at 120°C for 36h.
[0060] Add 2g of polyetheretherketone into a round bottom flask, pour 20mL of concentrated sulfuric acid into it, and keep stirring at room temperature for 24h to complete the sulfonation reaction. The obtained purple solution is slowly poured into ice water, and the polymer obtained after filtration is passed through Na with a mass concentration of 1%-5% (3%) 2 CO 3 The solution was washed several times (here 5 times) and then washed with water until the pH was neutral, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com