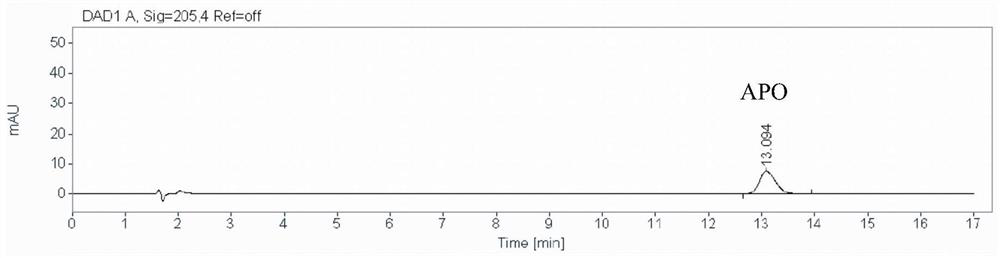
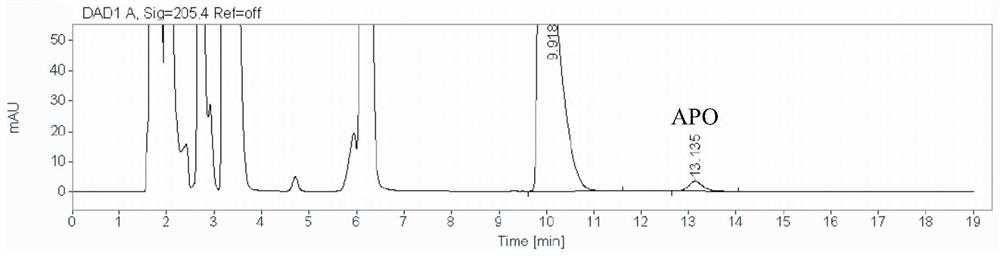
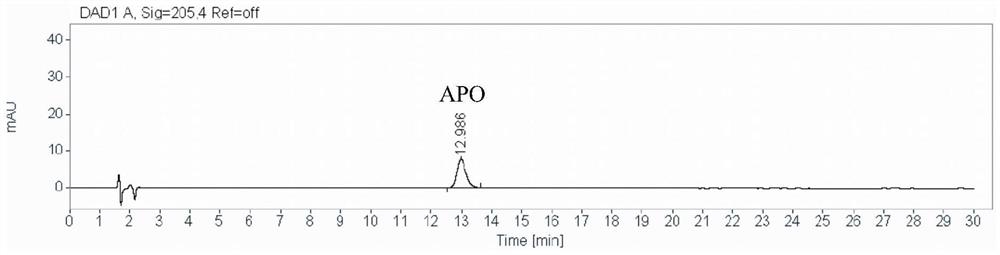
A method for detecting the content of 3-amino-2-piperidone in compound amino acid injection
A compound amino acid and piperidone technology, which is applied to measuring devices, instruments, scientific instruments, etc., can solve the problems of complex composition of compound amino acid injection, poor separation effect, long equilibration time of HILIC chromatographic column, etc., and achieve fast equilibrium and quantitative Low limit, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for detecting the content of 3-amino-2-piperidone in compound amino acid injection, comprising the steps of:
[0035] (1) Preparation of 3-amino-2-piperidone stock solution: Take 83.76 mg of the reference substance 3-amino-2-piperidone, put it in a 100mL volumetric flask, dissolve it with an appropriate amount of ultrapure water and dilute to the mark to prepare Obtain 3-amino-2-piperidone solution for subsequent use;
[0036] Preparation of 3-amino-2-piperidone intermediate solution: Precisely measure 1mL of 3-amino-2-piperidone stock solution in a 10mL volumetric flask, add ultrapure water to dilute to the mark and shake well;
[0037] Preparation of 3-amino-2-piperidone reference solution (abbreviated as APO ST): Accurately measure 1mL of 3-amino-2-piperidone intermediate solution in a 25mL volumetric flask, add ultrapure water to dilute to the mark and shake well. Prepare a 3-amino-2-piperidone reference substance solution with a concentration of 3.32 μg / m...
Embodiment 2
[0045] A method for detecting the content of 3-amino-2-piperidone in compound amino acid injection, comprising the steps of:
[0046] (1) Preparation of 3-amino-2-piperidone stock solution: Take 83.76 mg of reference substance 3-amino-2-piperidone, accurately weighed, place in a 100mL volumetric flask, dissolve and dilute with appropriate amount of ultrapure water To scale, make 3-amino-2-piperidone solution for subsequent use;
[0047] Preparation of 3-amino-2-piperidone intermediate solution: Precisely measure 1mL of 3-amino-2-piperidone stock solution in a 10mL volumetric flask, add ultrapure water to dilute to the mark and shake well;
[0048] Preparation of 3-amino-2-piperidone reference solution (abbreviated as APO ST): Accurately measure 1mL of 3-amino-2-piperidone intermediate solution in a 25mL volumetric flask, add ultrapure water to dilute to the mark and shake well. Prepare a 3-amino-2-piperidone reference substance solution with a concentration of 3.32 μg / mL;
...
Embodiment 3
[0056] A method for detecting the content of 3-amino-2-piperidone in compound amino acid injection, comprising the steps of:
[0057] (1) Preparation of 3-amino-2-piperidone stock solution: Take 83.76 mg of the reference substance 3-amino-2-piperidone, put it in a 100mL volumetric flask, dissolve it with an appropriate amount of ultrapure water and dilute to the mark to prepare Obtain 3-amino-2-piperidone solution for subsequent use;
[0058] Preparation of 3-amino-2-piperidone intermediate solution: Precisely measure 1mL of 3-amino-2-piperidone stock solution in a 10mL volumetric flask, add ultrapure water to dilute to the mark and shake well;
[0059] Preparation of 3-amino-2-piperidone reference solution (abbreviated as APO ST): Accurately measure 1mL of 3-amino-2-piperidone intermediate solution in a 25mL volumetric flask, add ultrapure water to dilute to the mark and shake well. Prepare a 3-amino-2-piperidone reference substance solution with a concentration of 3.32 μg / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com