Patents
Literature
30 results about "Argininic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Argininic acid is an organonitrogen compound and an organooxygen compound. It derives from a delta-amino acid.
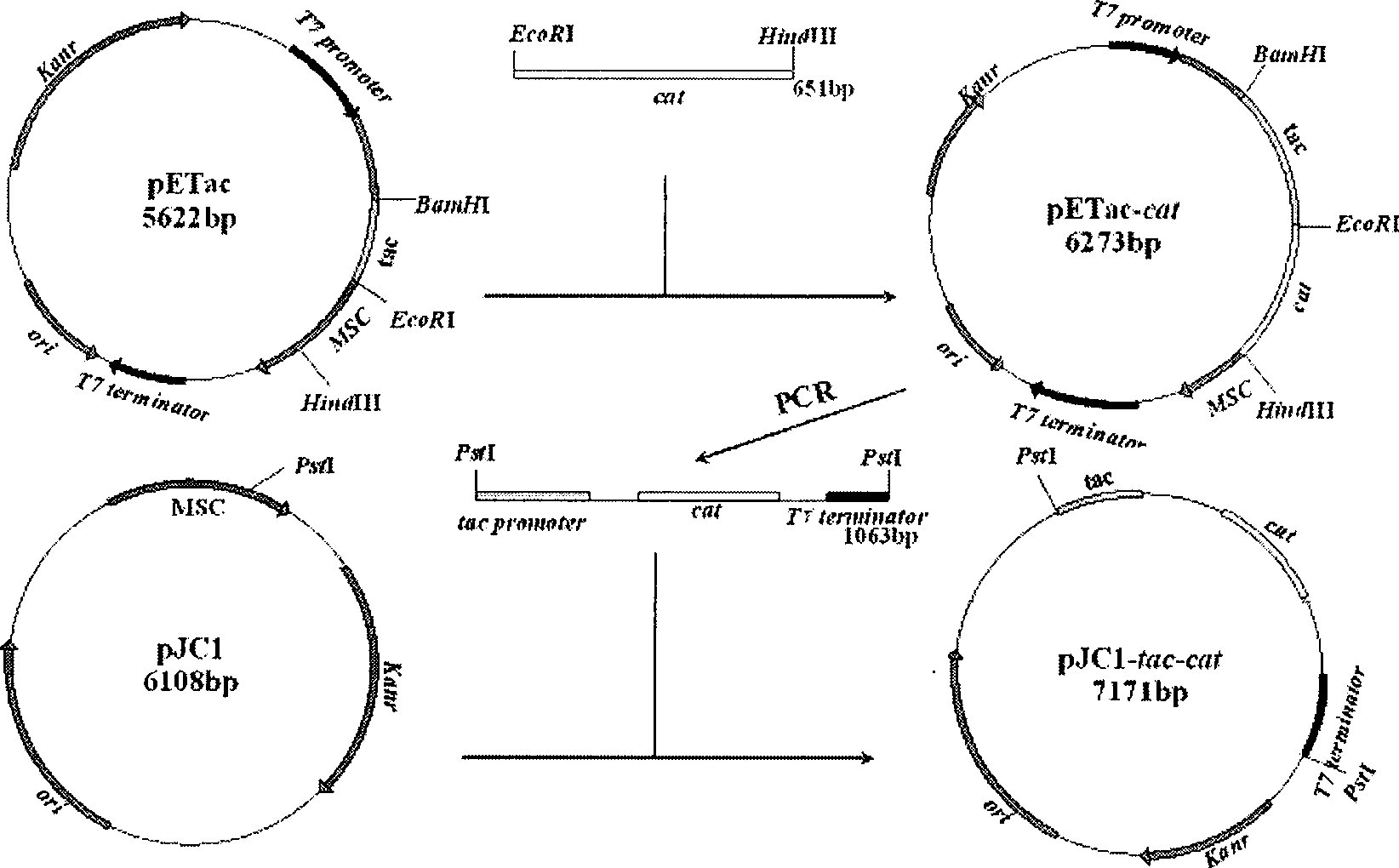
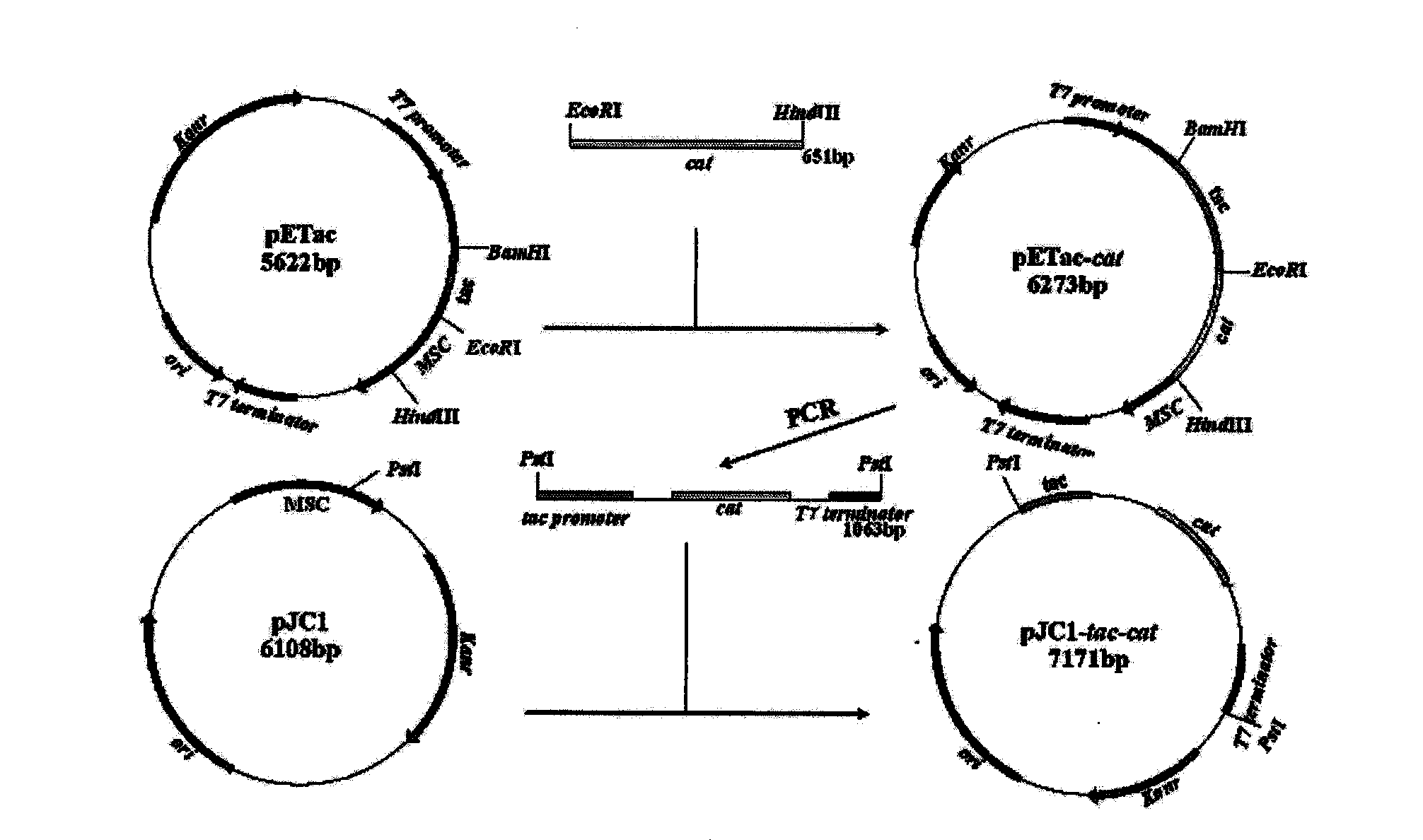
Recombinant corynebacterium crenatum for over expression of N-acetylglutamate kinase and application thereof
The invention relates to a recombinant corynebacterium crenatum to enhance the expression of N-acetyl glutamic acid kinase and the application thereof, which belongs to the technical field of gene engineering. The classification and nomenclature of the recombinant corynebacterium crenatum to enhance the expression of N-acetyl glutamic acid kinase is corynebacterium crenatum SYPA / pJC1-tac-argB with an access number of CCTCC NO: M 208133; The application is as follows: using a key enzyme N-acetyl glutamic acid kinase from corynebacterium crenatum pathway arginine to construct a corynebacterium crenatum expression vector pJC1-tac-argB; introducing the corynebacterium crenatum expression vector pJC1-tac-argB into corynebacterium crenatum SYPA by electrotransformation to obtain the recombinant corynebacterium crenatum SYPA / pJC1-tac-argB; and excessively expressing N-acetyl glutamic acid kinase, which greatly enhances the utilization rate of a precursor glutamic acid and allows the metabolic flow to flow to the arginine synthesis pathway, weakens the synthesis of proline, achieves the purpose of improving the output of arginine and the output of arginine is increased by 23.4 percent than C. crenatum SYPA.
Owner:JIANGNAN UNIV
Preparation method and application of feed additive N-carbamylglutamic acid
InactiveCN101759603ARaw materials are easy to getSimple methodUrea derivatives preparationOrganic compound preparationCarbamylglutamic acidArginine
The invention discloses a preparation method and application of feed additive N-carbamylglutamic acid. The steps of the preparation method are that: a. L-glutamic acid, methanol and sulfuric acid are mixed and react with each other, and triethylamine is used to regulate the pH value of the mixed solution to obtain L-alpha-methyl glutamate; the obtained L-alpha-methyl glutamate is added into the mixed solution of methanol and carbon disulfide, ammonia is fed in till the mixed solution is saturated, the obtained solution is placed under closed condition, and alpha-methyl ester-L-glutamic acid-N-amino-acid salt concentrated solution is obtained after concentration and ammonia removal; the concentrated solution is heated, acetic acid is added, the pressure is reduced to remove carbon disulfide to obtain crude N-carbamylglutamic acid, the crude N-carbamylglutamic acid is decolorized by active carbon and is recrystallized to obtain the finished product; and b. the N-carbamylglutamic acid is crushed and passes through a sieve, the obtained product is mixed with premix, and the obtained product is put and agitated in an agitator and is prepared into particles. The feed additive N-carbamylglutamic acid can promote the synthesis of endogenous arginine in bodies of piglets and sows. The method is simple, the operation is simple and convenient, the raw materials can be obtained easily and the cost is low. The produced feed can promote the synthesis of piglet proteins, relieve weanling stress of the piglets and improve the productivity of the sows.
Owner:INST OF SUBTROPICAL AGRI CHINESE ACAD OF SCI
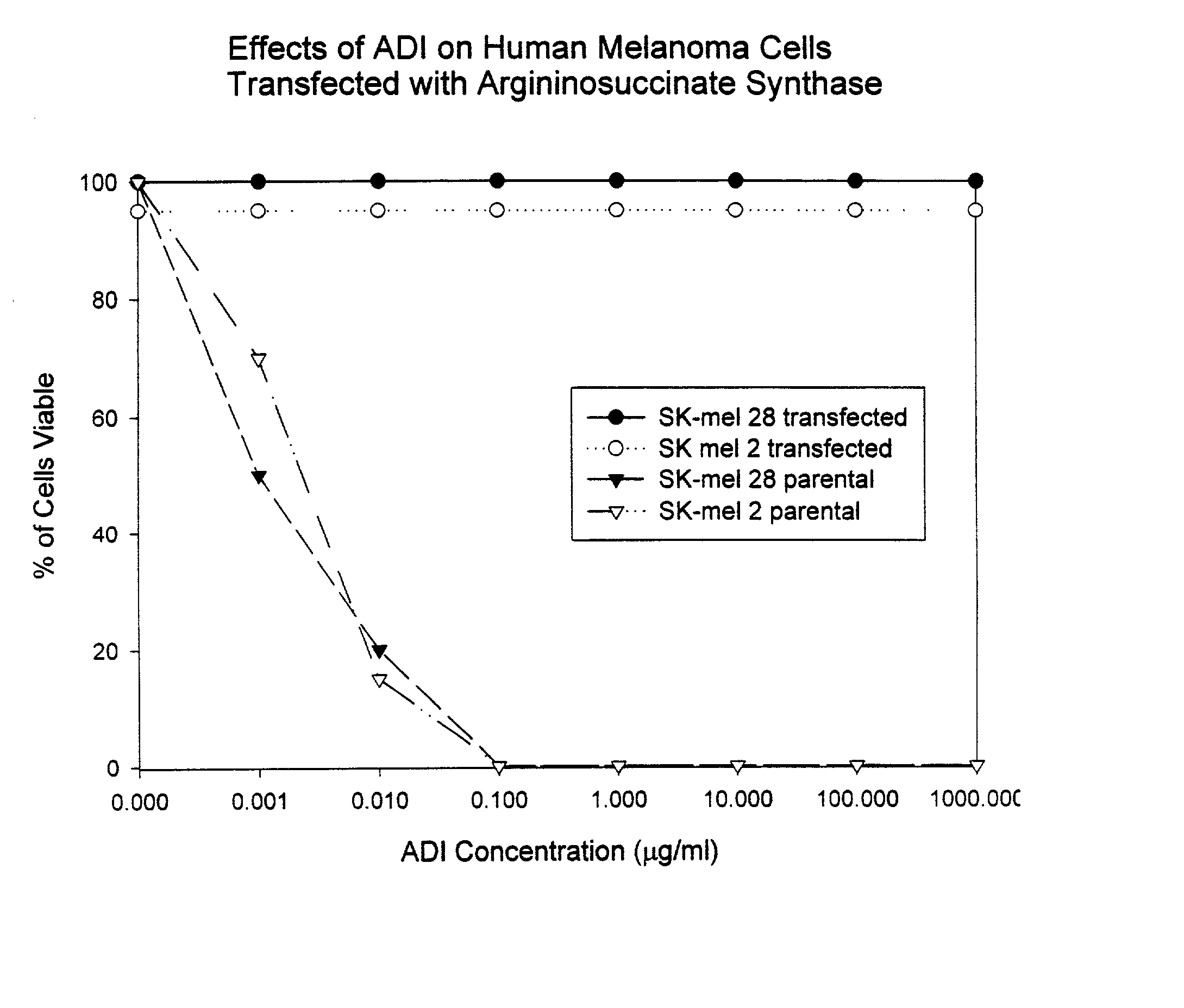
Methods for predicting sensitivity of tumors to arginine deprivation
InactiveUS20050063942A1Peptide/protein ingredientsMicrobiological testing/measurementAbnormal tissue growthUrea cycle enzymes
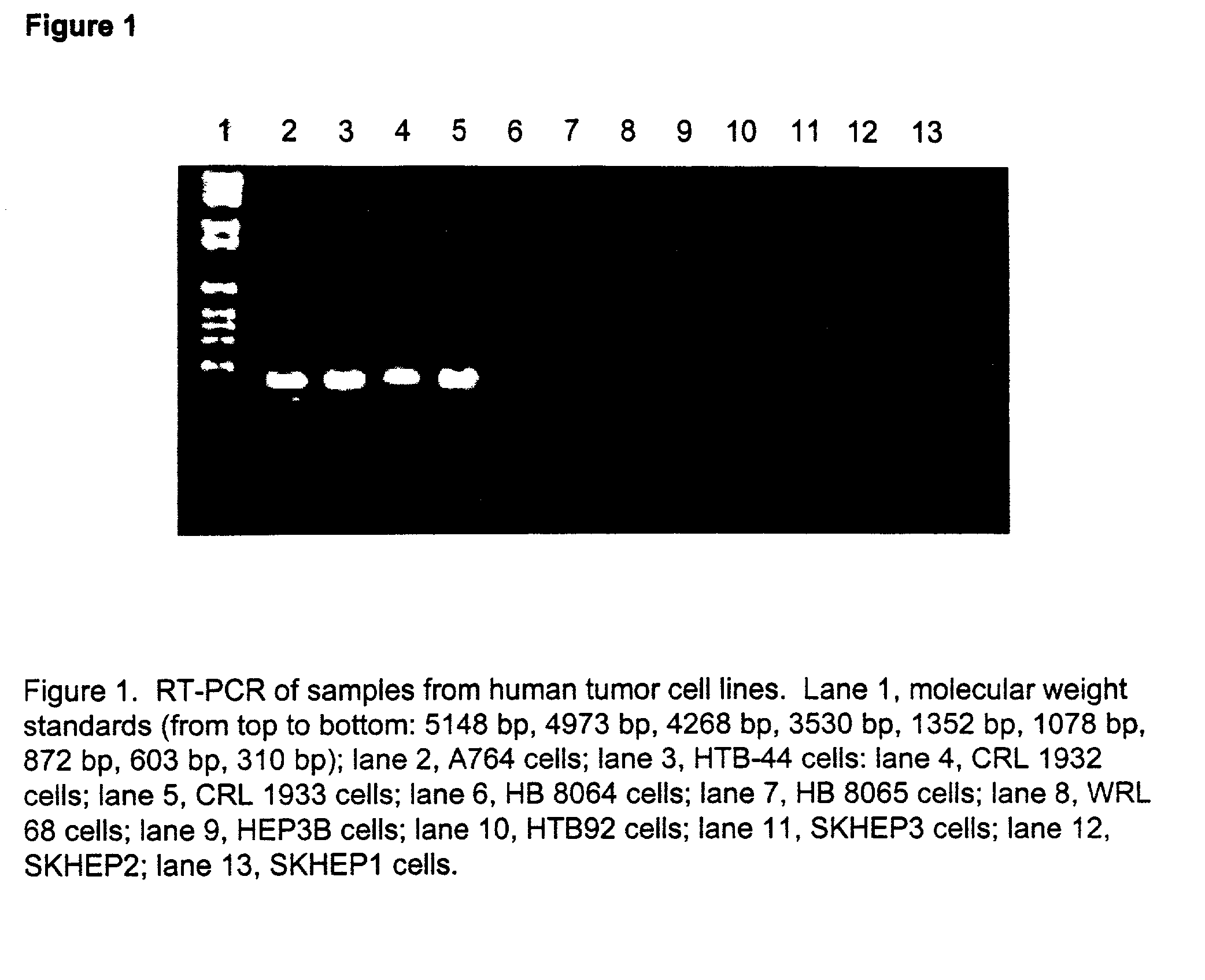
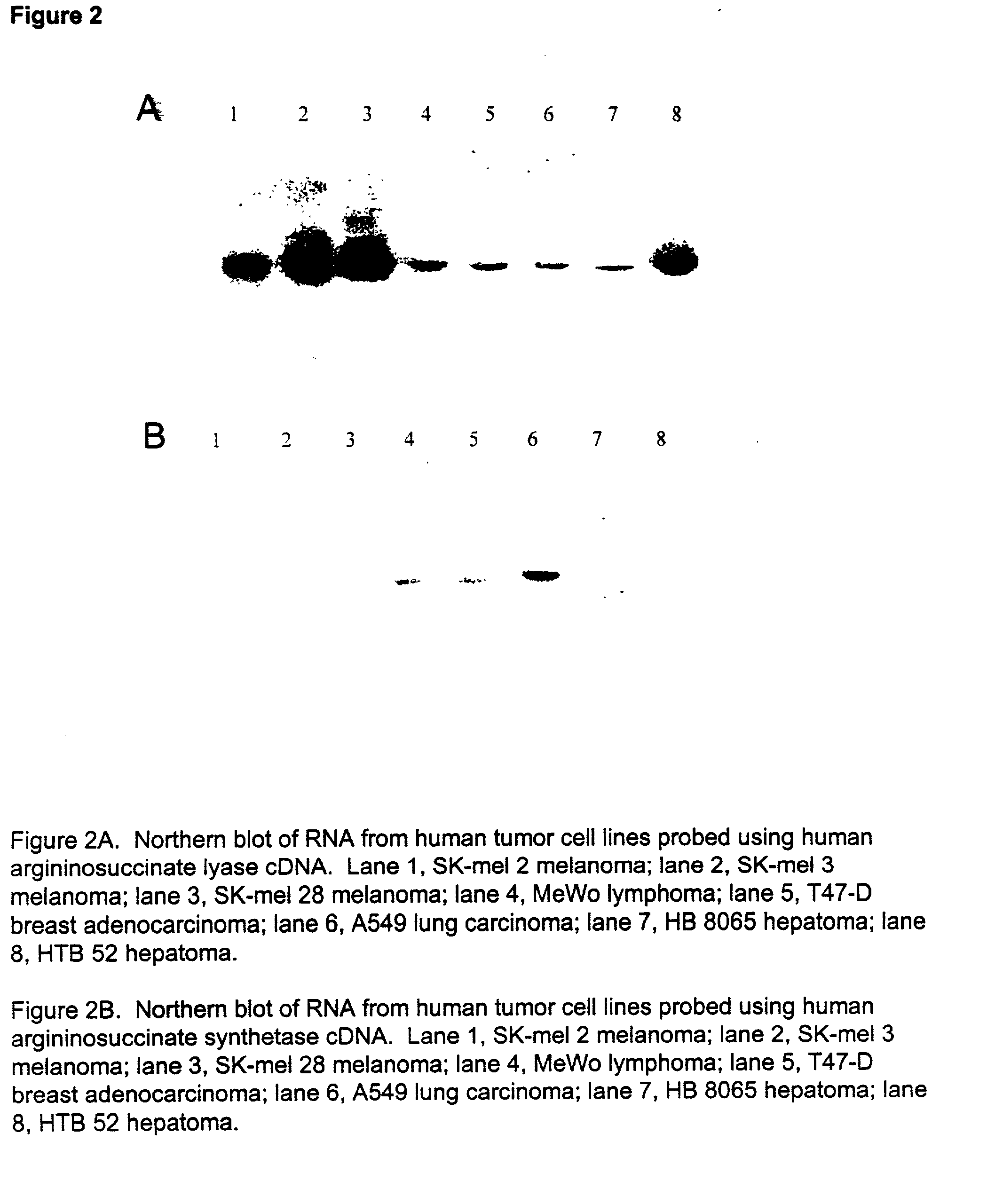
The present invention provides methods for determining which cancer patients are susceptible to arginine depletion therapy and methods for treating cancer. The present invention also provides methods for predicting the appropriateness of arginine deprivation therapy for a cancer patient. The methods generally comprise obtaining a tumor sample from the cancer patient and detecting the presence or absence of evidence of urea cycle enzyme expression in the tumor sample. The absence of evidence of urea cycle enzyme expression in the tumor sample is indicative of a cancer patient who is a candidate for arginine deprivation therapy, and the presence of evidence of urea cycle enzyme expression in said tumor sample is indicative of a cancer patient who is not a candidate for arginine deprivation therapy. Prior to, simultaneous with, or after testing the tumor sample, the method further comprises the steps of obtaining a non-cancerous sample from the cancer patient and detecting the presence or absence of evidence of urea cycle enzyme expression in the non-cancerous sample, wherein the absence of evidence of urea cycle enzyme expression in the non-cancerous sample and absence of evidence of urea cycle enzyme expression in the tumor sample is indicative of a cancer patient who is not a good candidate for arginine deprivation therapy, the presence of evidence of urea cycle enzyme expression in the non-cancerous sample and the absence of evidence of urea cycle enzyme expression in the tumor sample is indicative of a cancer patient who is a good candidate for arginine deprivation therapy, and the presence of evidence of urea cycle enzyme expression in the tumor sample is indicative of a cancer patient who is not a candidate for arginine deprivation therapy.
Owner:PHOENIX PHARMACOLOGICS
Dry Syrup Containing Loratadine
Dry syrup preparations comprising loratadine as a hydrophobic medicinal drug are provided. The loratadine dry syrup preparations can be produced using a cellulose material or an argininic acid salt together with sugar.
Owner:SCHERING CORP
New bacterial strain degrading polycyclic aromatic hydrocarbons with five rings or six rings, andacquiring method and application of same
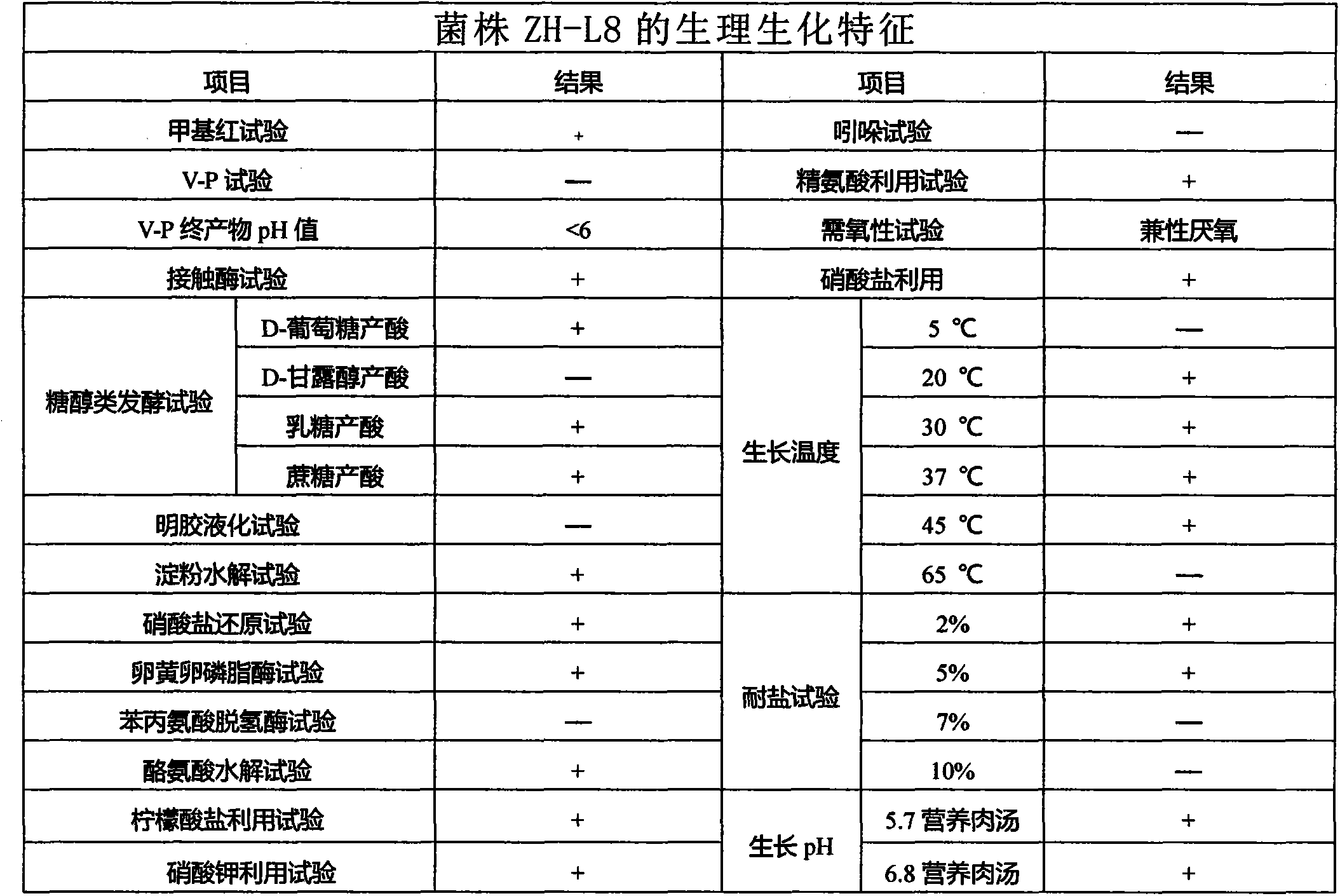
The embodiment in the invention provides a new bacterial strain degrading polycyclic aromatic hydrocarbons with five rings or six rings, and an acquiring method and application of the same, relates to the technical field of biological processing for environment pollutants, and the new bacterial strain is capable of efficiently degrading the polycyclic aromatic hydrocarbons with five rings or six rings. The new bacterial strain has the name of ZH-L8, the morphological characteristics comprise that a circle milk-white bacterium colony is grown on a solid medium and has a smooth edge; the new bacterial strain is in the shape of a rod when being observed under a microscope; the cell shows a Gram-positive rod shape, the later sides are parallel, and two ends are blunt; and the bacterium colony is circle, the edge is smooth, the bacterium colony is tightly adhered to the medium, and the bacterium colony is white and not transparent. The physiological biochemical characteristics comprise that the bacterium well grows in the temperature scope of 20-45 DEG C, grows well when pH is 5.7 and 6.8, has the salt-resistant concentration of 5%, and is methyl-red positive, contact-enzyme positive, v-p negative with the v-p solution final pH less than 6, arginine utilization positive, nitrate utilization positive, positive for D-glucose acid production, lactose acid production and cane sugar acid production, positive for starch hydrolysis, and the like.
Owner:HEBEI AGRICULTURAL UNIV.
Hyriopsis cumingii enzymolysis polypeptide, as well as preparation and application thereof
ActiveCN101503727AHigh in proteinEasy to storeHydrolysed protein ingredientsImmunological disordersArginineTert-leucine
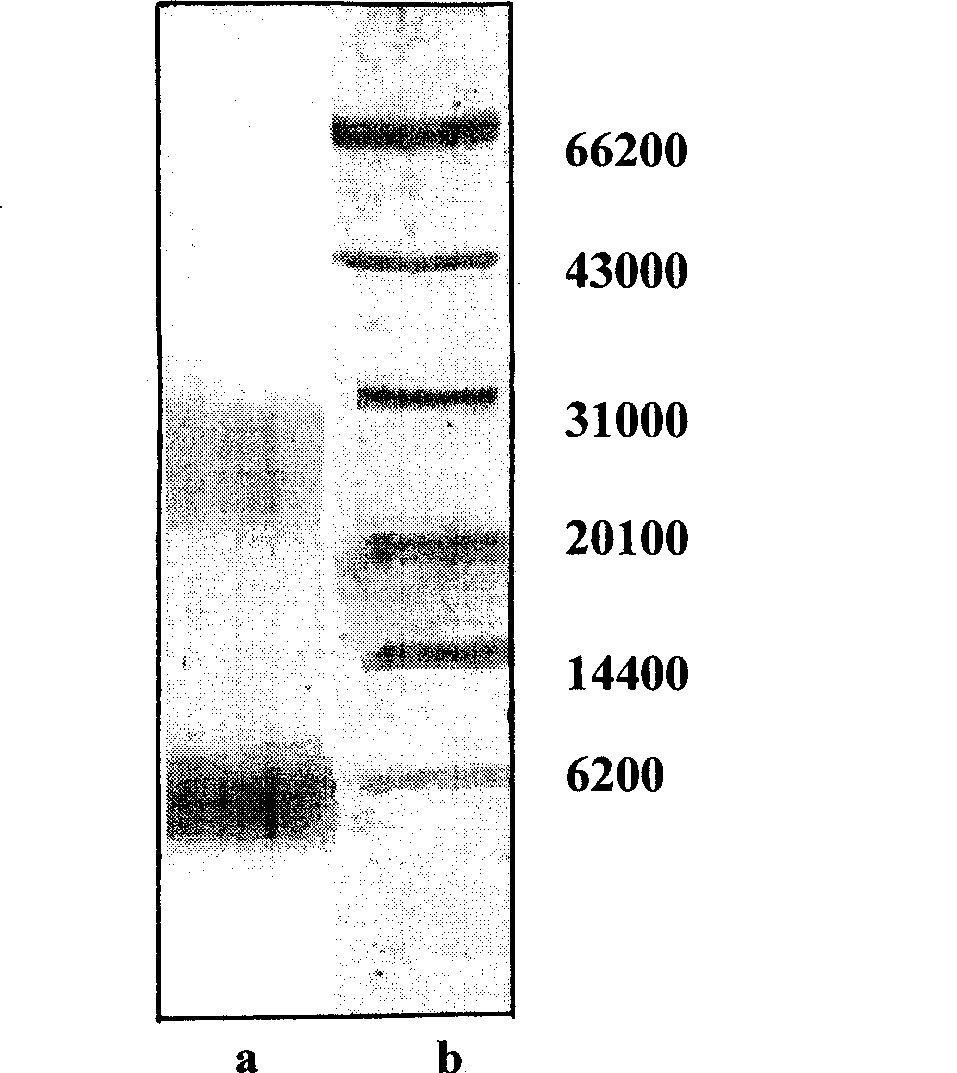
The invention provides a Hyriopsis cumingii enzymolysis polypeptide, preparation and application thereof. The enzymolysis method comprises the following steps that: fresh Hyriopsis cumingii meat is crushed and pulped, and added with water of which the weight is 5 to 6 times of that of the fresh Hyriopsis cumingii meat; the mixture is boiled and filtered; the precipitate is dried and degreased to obtain albumen powder of the Hyriopsis cumingii meat; the albumen powder of the Hyriopsis cumingii meat is soaked in water of which the weight is 6 to 8 times of that of the albumen powder of the Hyriopsis cumingii meat for 11 to 12 hours and subjected to superfine grinding and even pulping, and the pH value of the mixed solution is adjusted to between 8.0 and 9.0; based on the albumen content in the albumen powder of the Hyriopsis cumingii meat, 0.3 to 0.6 percent of alkali protease is added in the mixed solution for enzymolysis for 4 to 6 hours at a temperature of between 40 and 60 DEG C; the obtained supernatant is condensed and dried to obtain the Hyriopsis cumingii enzymolysis polypeptide; and by testing, the content of the total albumen of the Hyriopsis cumingii enzymolysis polypeptide is more than or equal to 80 percent, wherein the contents of asparagic acid, glutamic acid, leucine, lysine and arginine are more than or equal to 50 percent, and the molecular weight is mainly concentrated in two zones of 6,100Da and 26,200Da. By a test for culturing mouse lymphocytes in vitro, the Hyriopsis cumingii enzymolysis polypeptide can be used for preparing a medicament for promoting proliferation of the T lymphocute and a health care food for improving the immunity.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Caprine placental peptide-bone collagen polypeptide composition, mask liquid containing composition
InactiveCN108309919AMoisturizingEffective moisturizingCosmetic preparationsToilet preparationsArginineSkin elasticity
The invention discloses a caprine placental peptide-bone collagen polypeptide composition. The caprine placental peptide-bone collagen polypeptide composition is characterized by being prepared from the following components in percentage by mass: 1 to 3 percent of dipropylene glycol, 3 to 8 percent of glycerinum, 0.5 to 1 percent of glycerinum polyacrylate, 1 to 3 percent of xanthan gum, 0.5 to 1percent of panthenol, 1 to 3 percent of carbomer, 0.5 to 1 percent of nicotinamide, 0.5 to 1 percent of dipotassium glycyrrhizinate, 0.05 to 1.5 percent of hydrolyzed caprine placenta extract, 0.3 to0.5 percent of caprine placental peptide, 0.2 to 0.3 percent of bone collagen popypeptide, 0.05 to 1.5 percent of hydrolyzed collagen, 1 to 3 percent of aloe barbadensis leaf juice, 3 to 6 percent ofchamomilla recutita flower extract, 0.5 to 1 percent of beta-glucosan, 0.5 to 1 percent of arginine, 0.05 to 1.5 percent of hyaluronic acid, 0.5 to 1 percent of oligopeptide-1, 0.5 to 1 percent of palmitoyl pentapeptide-4, 0.5 to 1 percent of palmitoyl tripeptide-5, 1 to 3 percent of saccharide isomerate, 1 to 3 percent of papain, 0.5 to 1 percent of tridecanol polyether-9, 2 to 5 percent of melissa officinalis flower / leaf / stem water, 1 to 3 percent of rosemary leaf water, 0.05 to 0.5 percent of methylparaben, 0.05 to 0.5 percent of EDTA disodium, 0.01 percent of essence and the balance of water. By adopting the caprine placental peptide-bone collagen polypeptide composition, the caprine placental peptide-bone collagen polypeptide composition is prepared into the mask liquid, the skin elasticity is enhanced, moisture is kept effectively, small wrinkles on the skin can be tightened and softened, so that the skin is smooth.
Owner:广东肽世家生物科技有限公司
Formaldehyde scavenger and preparation method thereof
ActiveCN110860179ASolve the problemsNo dust problemGas treatmentOther chemical processesFiberCarbon fibers
The invention relates to the technical field of formaldehyde air pollution treatment, in particular to a composite formaldehyde scavenger of activated carbon fiber loaded amino acid and a preparationmethod thereof. The formaldehyde scavenger is activated carbon fiber loaded amino acid, the mass ratio of the amino acid to the activated carbon fiber is 0.001-0.4:1, the amino acid comprises at leastone of glycine, alanine, arginine, lysine, proline and serine, and the activated carbon fiber carrier is activated carbon fiber honeycomb filter cotton or activated carbon fiber felt. The preparationmethod includes: firstly, dissolving amino acid in water to obtain a solution with a concentration of 0.01-20%; and putting an activated carbon fiber product into the solution, conducting soaking for0.5-24h for adsorption loading, then taking the activated carbon fiber product out of the solution, and conducting drying to obtain the formaldehyde scavenger. The formaldehyde scavenger provided bythe invention has no dust problem, provides a barrier-free network type carrier material with a large specific surface area for amino acid, can be for repeated loading, and is efficient, safe, simpleand convenient.
Owner:上海置顶环境科技有限公司
Lactobacillus fermentum capable of degrading arginine and urea simultaneously
ActiveCN106479923APromote degradationBacteriaMicroorganism based processesArginineLactobacillus fermentum
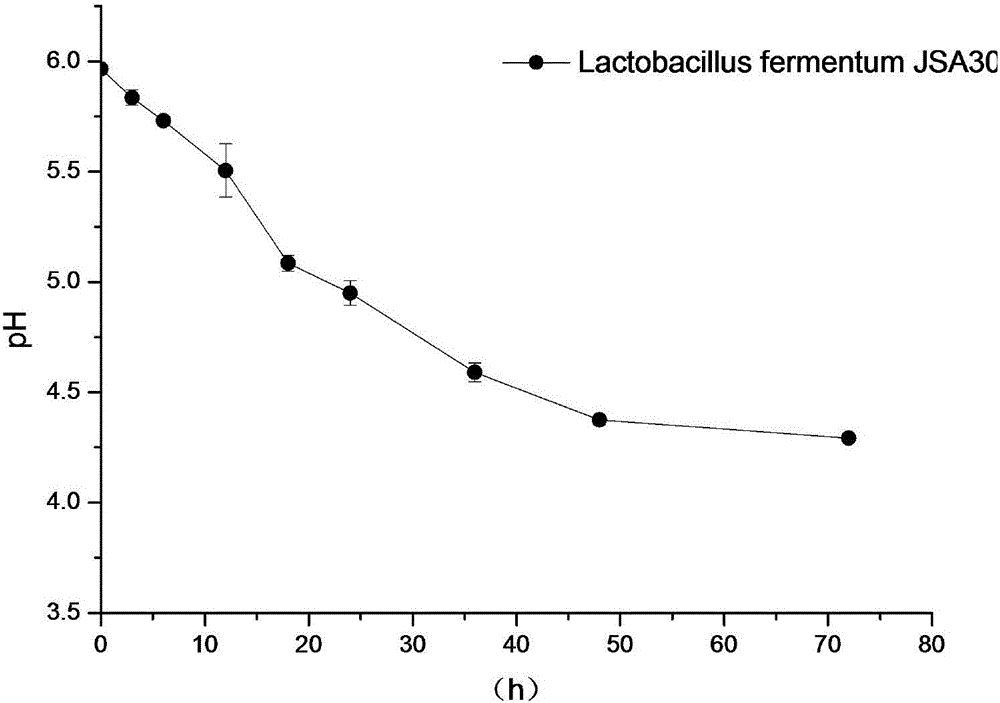
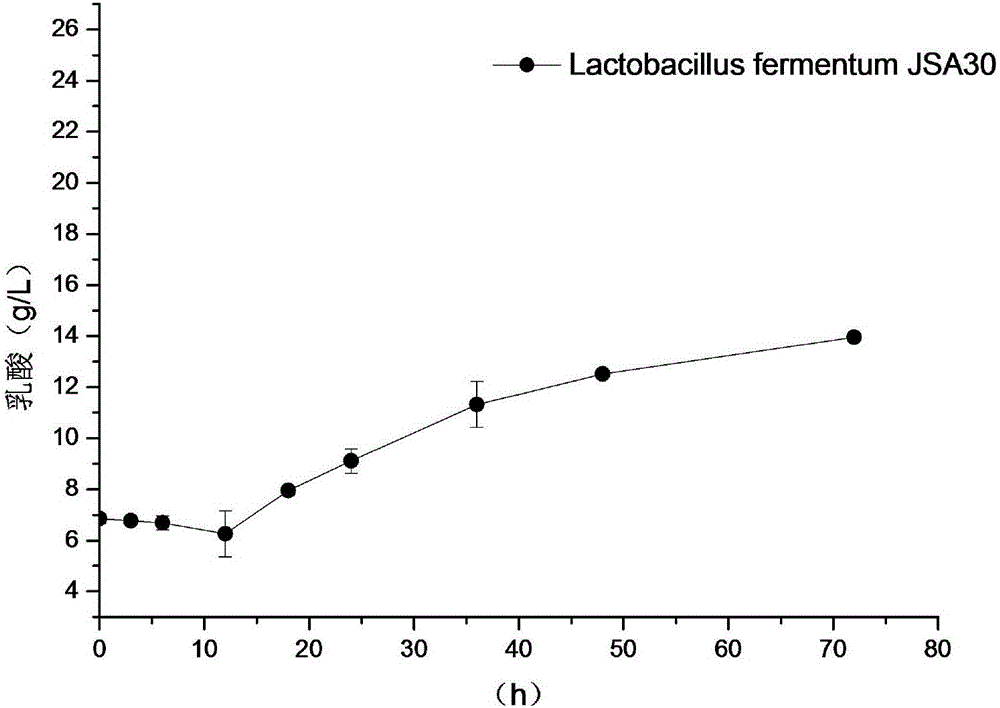
The invention, which belongs to the field of the microbial technology, discloses lactobacillus fermentum capable of degrading arginine and urea simultaneously. The capability of the lactobacillus fermentum for degrading urea reaches up to 1.96mmol.L<-1>; and the arginine degrading capability of the lactobacillus fermentum reaches up to 0.86mmol.L<-1> and only very little urea is generated. Meanwhile, metabolism of acids, alcohol, ketone, and phenols and the like can be realized and thus important flavor compounds can be provided for Baijiu. Anaerobic digestion is carried out for 2 days at a temperature of 30 DEG C in an MRS culture medium and then the operation enters a stable stage, wherein the cell concentration can meet a condition: OD600=2.4, the fermentation broth pH reaches about 4.3, the lactic acid content is about 12g / L; and after fermentation for 3 days, the acetic acid yield can reach 6g / L.
Owner:JIANGNAN UNIV
Method for reducing contents of urea and ethyl carbamate in rice wine brewing process
ActiveCN110656008AInhibition loopReduce degradationMicroorganism based processesAlcoholic beverage preparationGlutelinNitrogen source
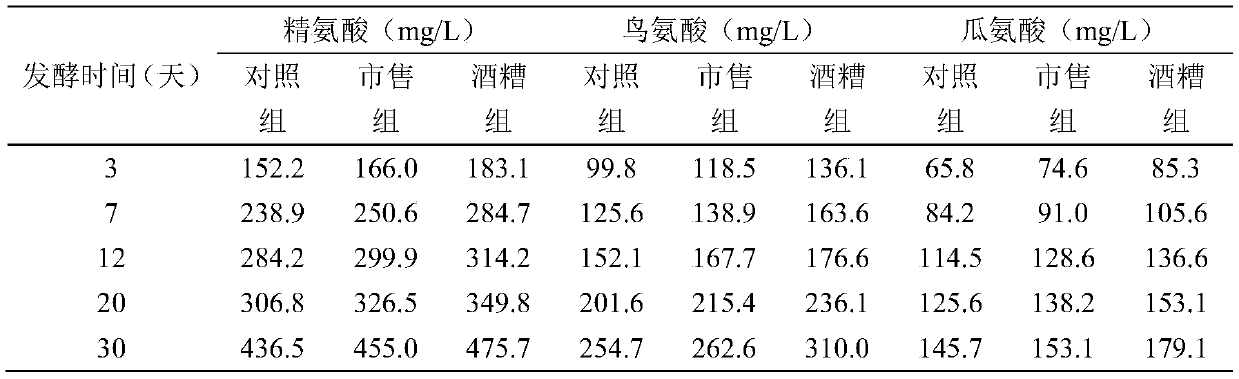
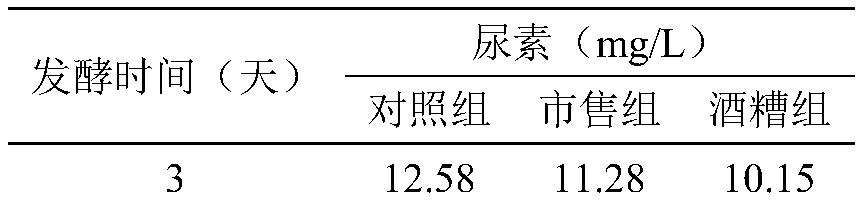
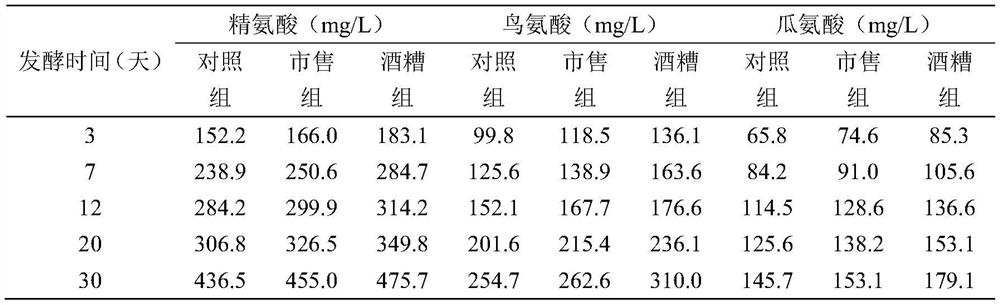
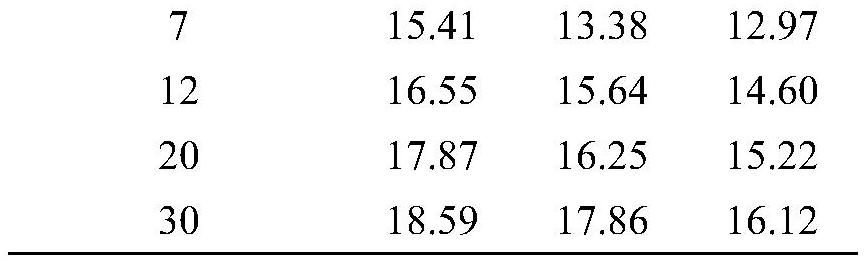
The invention discloses a method for reducing contents of urea and ethyl carbamate in a rice wine brewing process. The method comprises the following steps: adding distillers' grain glutelin into a rice wine brewing system, and performing fermentation, wherein the addition amount of the distillers' grain glutelin is 0.5-8g / 100g in terms of the mass of raw material rice. As discarded distillers' grains of rice wine are reused, glutelin is effectively extracted, and the distillers' grain glutelin is added at an initial fermentation stage of the rice wine, constitution of a nitrogen source utilized by yeasts in a wine brewing process can be regulated and controlled, urea circulation can be effectively inhibited, degradation of arginine can be reduced, and thus formation of EC (ethyl carbamate) can be reduced.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Medicinal nutrition powder for patient with sarcopenia and preparation method
InactiveCN111387484AImprove daily energy needsGuaranteed concentrationFood ingredient as thickening agentVitamin food ingredientsBiotechnologyHydrolysate
The invention discloses medicinal nutrition powder for a patient with sarcopenia and a preparation method and relates to the technical field of medicinal foods. The medicinal nutrition powder comprises the following components in parts by weight: 98.5-788 parts of protein substances, 64-513 parts of fatty substances, 240-1920 parts of carbohydrate, 14-112 parts of mineral, 4.2-33.6 parts of compound vitamin, 0.4-3.2 parts of taurine, 0.5-4 parts of single double glyceride fatty acid ester, 0.09-0.72 part of nucleotide, 0.06-0.48 part of L-carnitine, 9-70 parts of a thickening agent, 1.5-9 parts of concentrated vanilla and 0.28-2.2 parts of aspartame; and the protein substances comprise 35-325 parts of lactalbumin powder, 30-240 parts of soybean protein isolate, 25-200 parts of whole milk powder, 0.5-4.5 parts of bovine colostrum, 5-40 parts of soybean oligopeptide, 15-120 parts of lact albumin hydrolysate, 5-40 parts of fish collagen peptide, 4-38 parts of marine fish oligopeptide, 2.5-20 parts of L-arginine and 3-24 parts of L-leucine. For the nutrition requirement for the sarcopenia, the medicinal nutrition powder can be used as a single nutrient source to meet the nutrition requirement of people with the sarcopenia, and the sarcopenia is reversed or prevented.
Owner:特康药业集团有限公司
Rabdosia rubescens (Hemsl.) Hara callus induction medium capable of increasing content of oridonin and rosmarinic acid
ActiveCN111134020AGuaranteed survival rateShort generation cyclePlant tissue cultureHorticulture methodsRosmarinusPolyamine
The invention relates to a Rabdosia rubescens (Hemsl.) Hara callus induction medium capable of increasing the content of oridonin and rosmarinic acid. The medium is characterized by comprising the following components: in percent by mass, 3%-5% of an MS medium, 2%-2.5% of 1,5-dehydrated-D-glucitol, 2%-3% of a mixture of KNO3 and (NH4)2SO4, 0.1%-2% of plant polyamine, 2%-5% of oxymatrine, 0.002%-0.004% of ethyl lauroyl arginate hydrochloride, 0.3%-0.5% of agar and the balance deionized water. The medium has the advantage of simultaneously increasing the content of oridonin and rosmarinic acid in the callus of Rabdosia rubescens (Hemsl.) Hara.
Owner:PROYA COSMETICS
Dry syrup containing loratadine
Dry syrup preparations comprising loratadine as a hydrophobic medicinal drug are provided. The loratadine dry syrup preparations can be produced using a cellulose material or an argininic acid salt together with sugar.
Owner:SCHERING CORP
Application of arginine in preparation of preservative for improving generation capacity and biocontrol effect of Metschnikowia citriensis pulcherriminic acid
ActiveCN112586559AImprove the effectiveness of biological controlSolve pollutionFruit and vegetables preservationClimate change adaptationBiotechnologyMicrobial pesticide
The invention discloses an application of arginine in preparation of a preservative for improving the pulcherriminic acid production capacity of Metschnikowia citriensis, and a method for improving the pulcherriminic acid production capacity of the Metschnikowia citriensis by using the arginine. The invention further provides application of the arginine in preparation of a preservative for improving the biological prevention and treatment effect of the Metschnikowia citriensis on postharvest diseases of fruits, and a method for improving the biological prevention and treatment effect of the Metschnikowia citriensis on the postharvest diseases of the fruits by utilizing the arginine. The effect is clear and remarkable, the problems of agricultural residues and environmental pollution causedby the use of pesticide can be effectively solved, good application potential is realized in the aspect of biological prevention and treatment of the postharvest diseases of the fruits, and meanwhile, an excellent method is provided for researching and developing microbial pesticides and enhancing the efficacy of the microbial pesticides.
Owner:SOUTHWEST UNIVERSITY
Method for detecting content of 3-amino-2-piperidone in compound amino acid injection
ActiveCN111337620AQuick balanceStrong specificityComponent separationAmino Acid InjectionIon-exchange resin
The invention relates to a method for detecting the content of 3-amino-2-piperidone, in particular to a method for detecting the content of 3-amino-2-piperidone in a compound amino acid injection. According to the invention, the content of 3-amino-2-piperidone in the compound amino acid injection is detected by high performance liquid chromatography; chromatographic conditions overcome the defectsthat a C18 chromatographic column is weak in retention and an amino column and an HILIC chromatographic column are difficult to balance, ion pair chromatography is poor in reproducibility and the like. The method has the advantages of fast chromatographic column balance, extremely good specificity, high sensitivity, good accuracy, good reproducibility, low quantification limit and the like, the method is simple to operate and fast in analysis, the diluted sample is directly separated and analyzed on line by using the strong cation exchange chromatographic column, and the method is simpler andfaster compared with a traditional method for manually treating the sample by using cation exchange resin. The detection method can be used for qualitative or quantitative analysis of 3-amino-2-piperidone in other amino acid products or ornithine hydrochloride, arginine and other bulk drugs.
Owner:费森尤斯卡比华瑞制药有限公司
Whitening cream and preparation method thereof
PendingCN113975215AEven skin toneRemove acne marks and spotsCosmetic preparationsToilet preparationsBiotechnologyGlycerol
The invention discloses whitening cream and a preparation method thereof, and relates to the technical field of whitening cosmetics. The whitening cream is prepared from the following raw material components in parts by weight: 31.95 to 86.88 parts of water; 1.1-7 parts of an emulsifier; 1 to 5 parts of butanediol; 1 to 5 parts of 1, 2-pentanediol; 1 to 5 parts of glyceryl polyether-26; 1 to 5 parts of panthenol; 1 to 5 parts of squalane; 1-5 parts of simmondsia chinensis seed oil; 1-5 parts of caprylic / capric triglyceride; 1 to 5 parts of isononyl isononanoate; 1 to 3 parts of hydroxyethyl piperazine ethanesulfonic acid; 0.5 to 4 parts of ascorbyl tetraisopalmitate; 0.05 to 0.1 part of glabridin; 0.5 to 2 parts of trehalose; 0.5 to 1 part of p-hydroxyacetophenone; 0.5 to 1 part of 1, 2-hexanediol; 0.5-2 parts of a ginger root extract solution; 0.1 to 1 part of beta-glucan; 0.05 to 0.5 part of carbomer; 0.05 to 0.5 part of xanthan gum; 0.05 to 5 parts of arginine; 0.1 to 0.5 part of dipotassium glycyrrhizinate; 0.1 to 0.3 part of allantoin; 0.01 to 0.1 part of sodium hyaluronate; 0.01 to 0.05 part of disodium EDTA; The emulsifying agent is prepared from an emulsifying agent Olivem 1000, an emulsifying agent Tego Core SE 121 and an emulsifying agent Tego Core 450; the ginger root extract solution is prepared from bisabolol and a ginger root extract.
Owner:广州梵之容化妆品有限公司
A kind of polypeptide and its synthesis method
ActiveCN111574591BLong-acting internal circulation timeImprove in vivo stabilityPeptide/protein ingredientsPeptide preparation methodsDisulfide bondingChemical synthesis
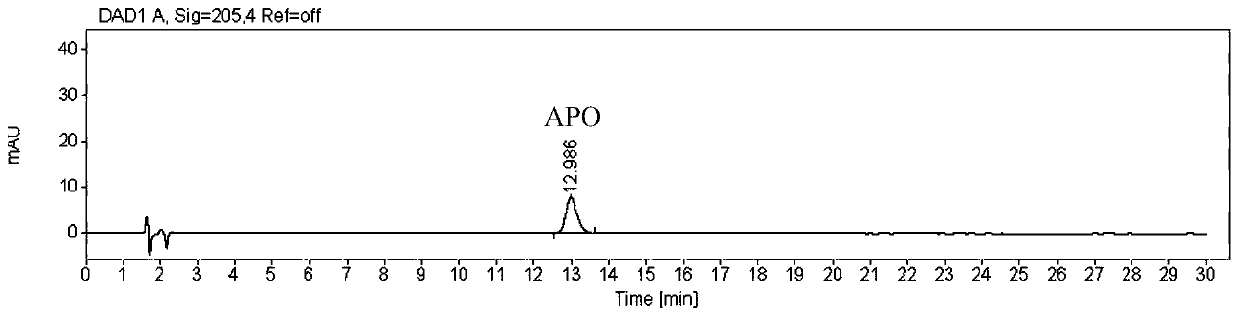
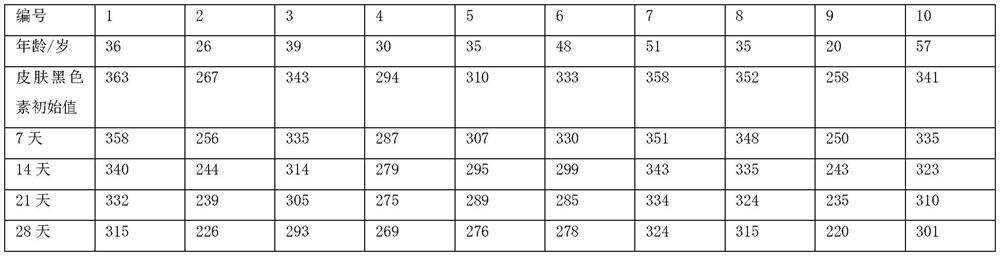
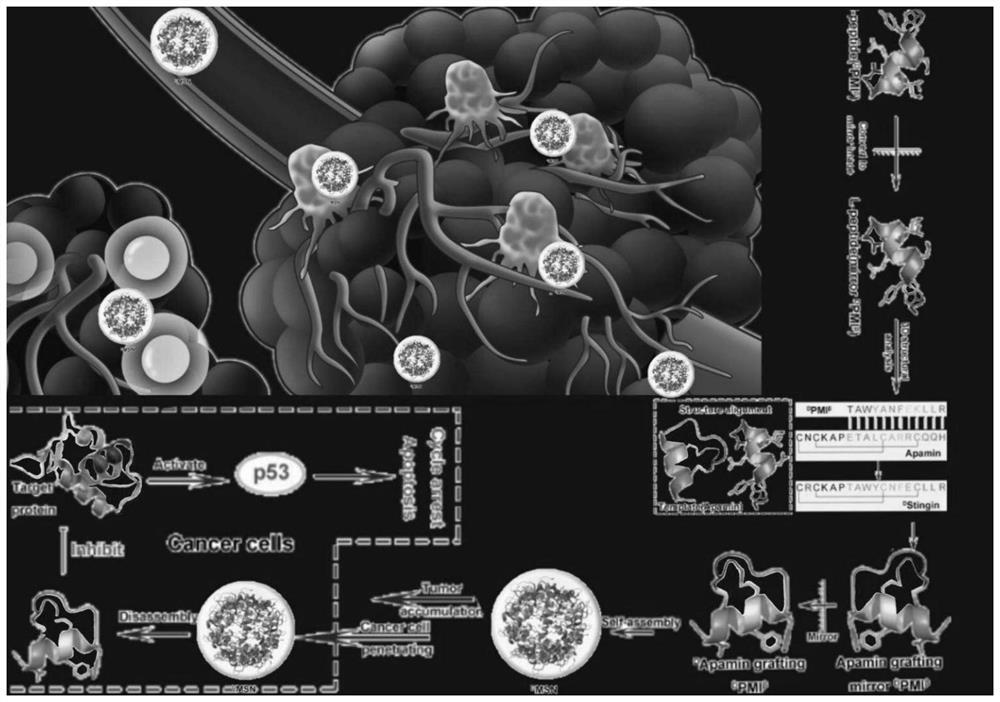
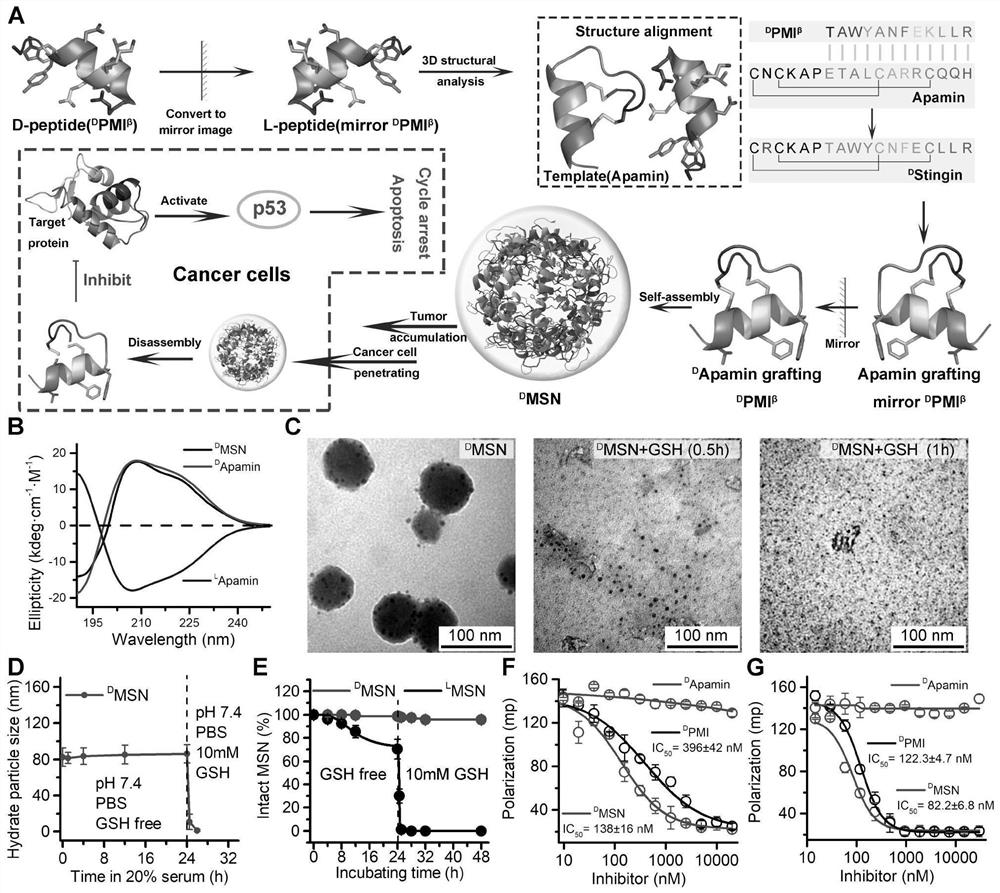
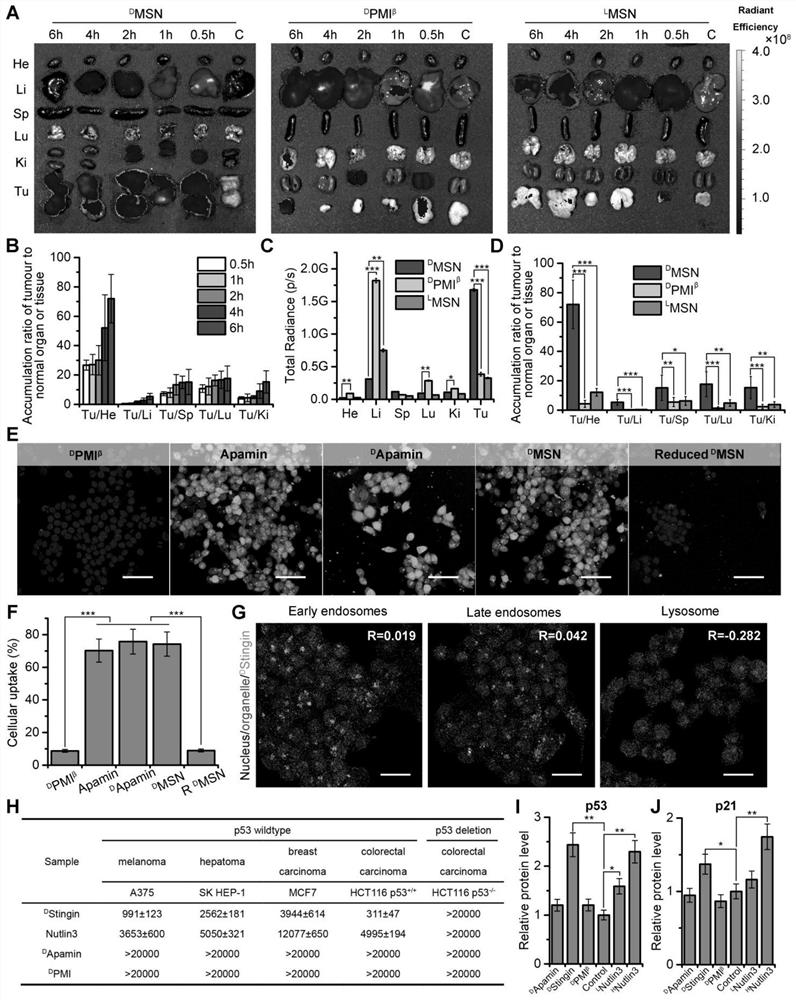
The invention discloses a polypeptide and a synthesis method thereof. The amino acid sequence of the polypeptide is shown in SEQ ID NO.1; the synthesis method of the polypeptide consists of the following steps: D PMI β Flip along the X axis with Cartesian coordinates to get the mirror counterpart L PMI β ;Will L PMI β Topologically grafted onto the α-helical region of Apamin, thereby getting the grafted L PMI β Apamin; will L PMI β Apamin is converted to D PMI β Apamin; will D PMI β The asparagine at position 2 in the Apamin ring is mutated to arginine, which self-assembles into D-enantiomer small protein supramolecular nanoparticles under the action of hydrophobic interactions and hydrogen bonds; after two-step disulfide bond oxidation, The target peptide is then chemically synthesized by Fmoc D MSN, target peptide D The amino acid sequence of MSN is shown in SEQ ID NO.1; the polypeptide is used to inhibit the growth of cancer cells; the polypeptide of the present invention has a long-acting circulation time in the body, and compared with the traditional small molecule drug of this target, the polypeptide drug has strong specificity , high targeting, and less side effects in the body.
Owner:陕西未来多肽生物科技有限公司
Method for producing 1, 4-butanediamine by fermenting xylose and xylose-containing hydrolysate
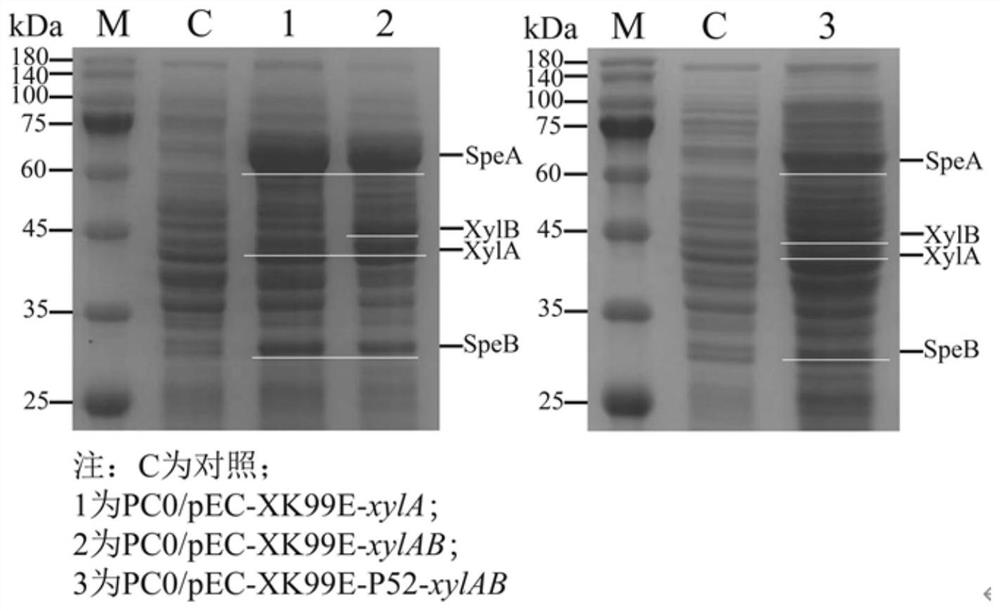
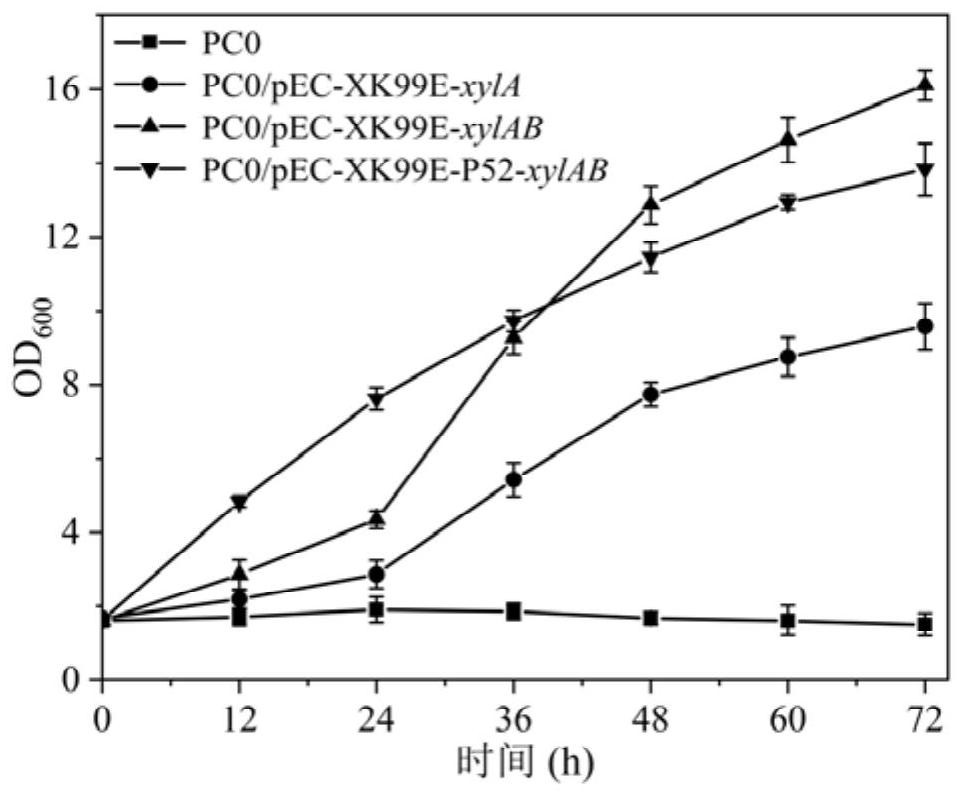
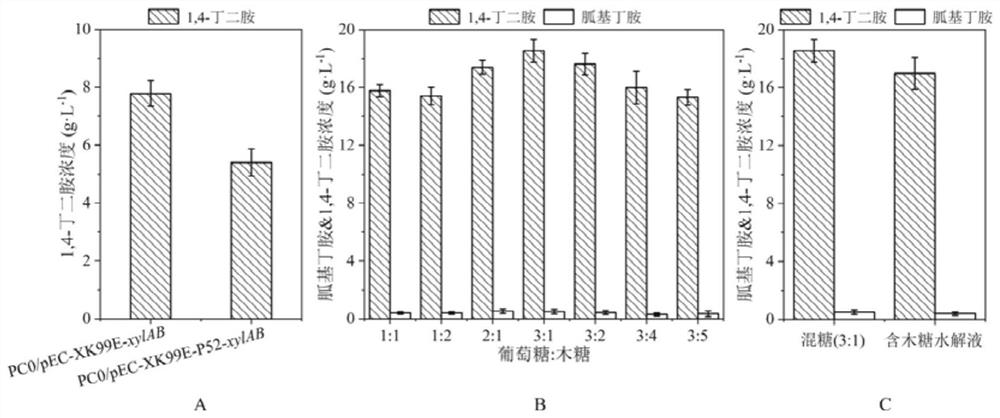
PendingCN114774342AIncrease carbon sourceThe result is obviousBacteriaHydrolasesEscherichia coliHydrolysate
The invention discloses a method for producing 1, 4-butanediamine by fermenting xylose and xylose-containing hydrolysate, and belongs to the technical field of genetic engineering. According to the invention, a shuttle plasmid pEC-XK99E between Escherichia coli and Corynebacterium crenatum is adopted, and a xylose isomerase and xylulokinase coding gene cluster derived from Escherichia coli K-12 is expressed in a previously constructed 1, 4-butanediamine high-yield strain PC0. A shake flask fermentation result shows that the original bacterium PC0 does not have the capability of metabolizing and utilizing the xylose, but the recombinant corynebacterium crenatum has the capability, so that the 1, 4-butanediamine can be directly produced by directly utilizing the xylose and xylose-containing hydrolysate. And finally, fermenting in a 5-L fermentation tank for 72 hours to accumulate 33.4 g.L <-1 >, 4-butanediamine. The method for producing the 1, 4-butanediamine has the advantages of high efficiency and low content of byproducts of L-arginine, agmatine and other heteroacids, and the separation and purification of the product 1, 4-butanediamine in the later stage are easier.
Owner:JIANGNAN UNIV
A method for reducing urea and ethyl carbamate content in rice wine brewing process
ActiveCN110656008BInhibition loopReduce degradationAlcoholic beverage preparationMicroorganism based processesGlutelinNitrogen source
Owner:ZHEJIANG FORESTRY UNIVERSITY
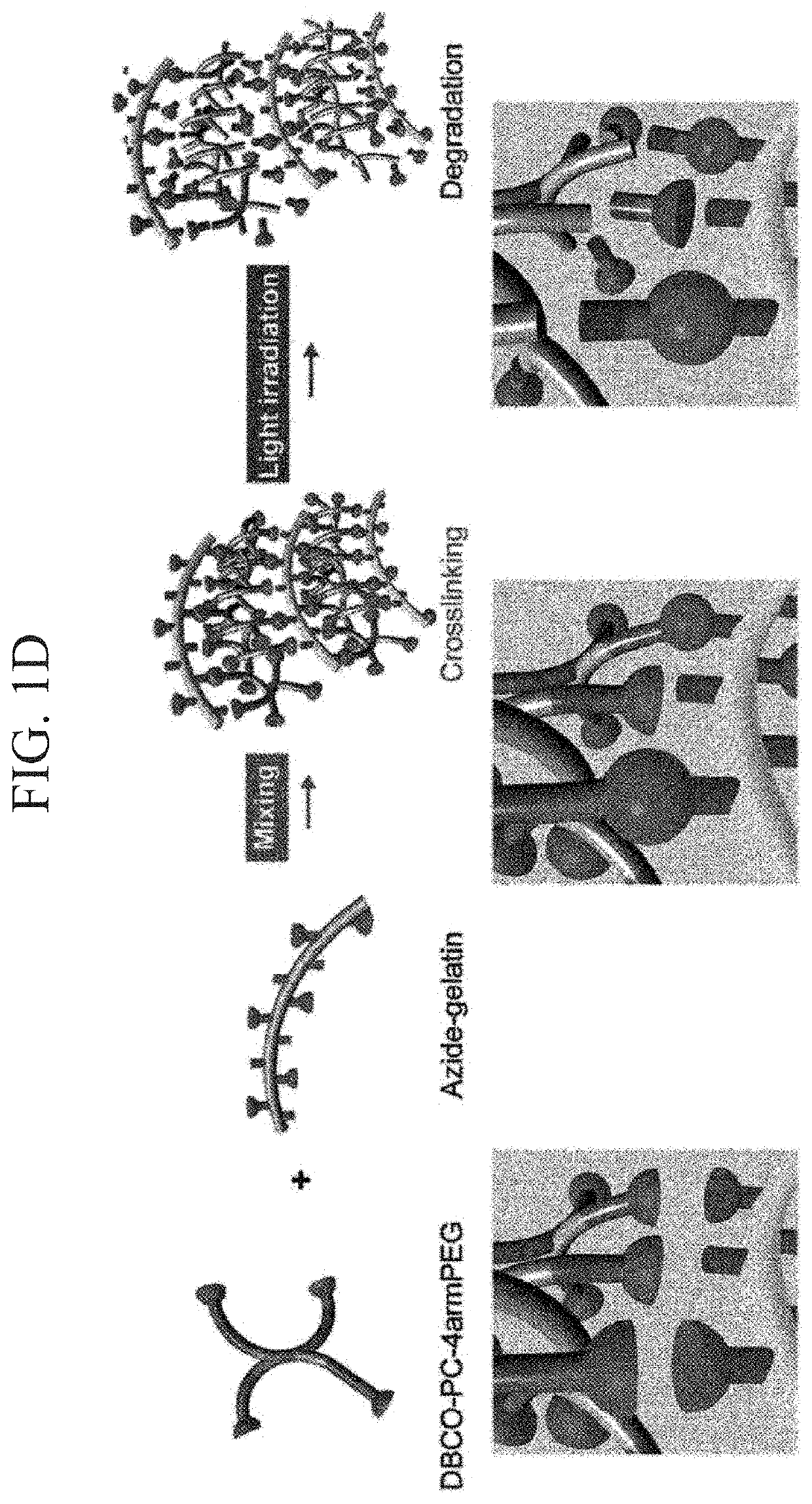
Photodegradable hydrogel, culture device, method for forming tissue, and method for separating cells
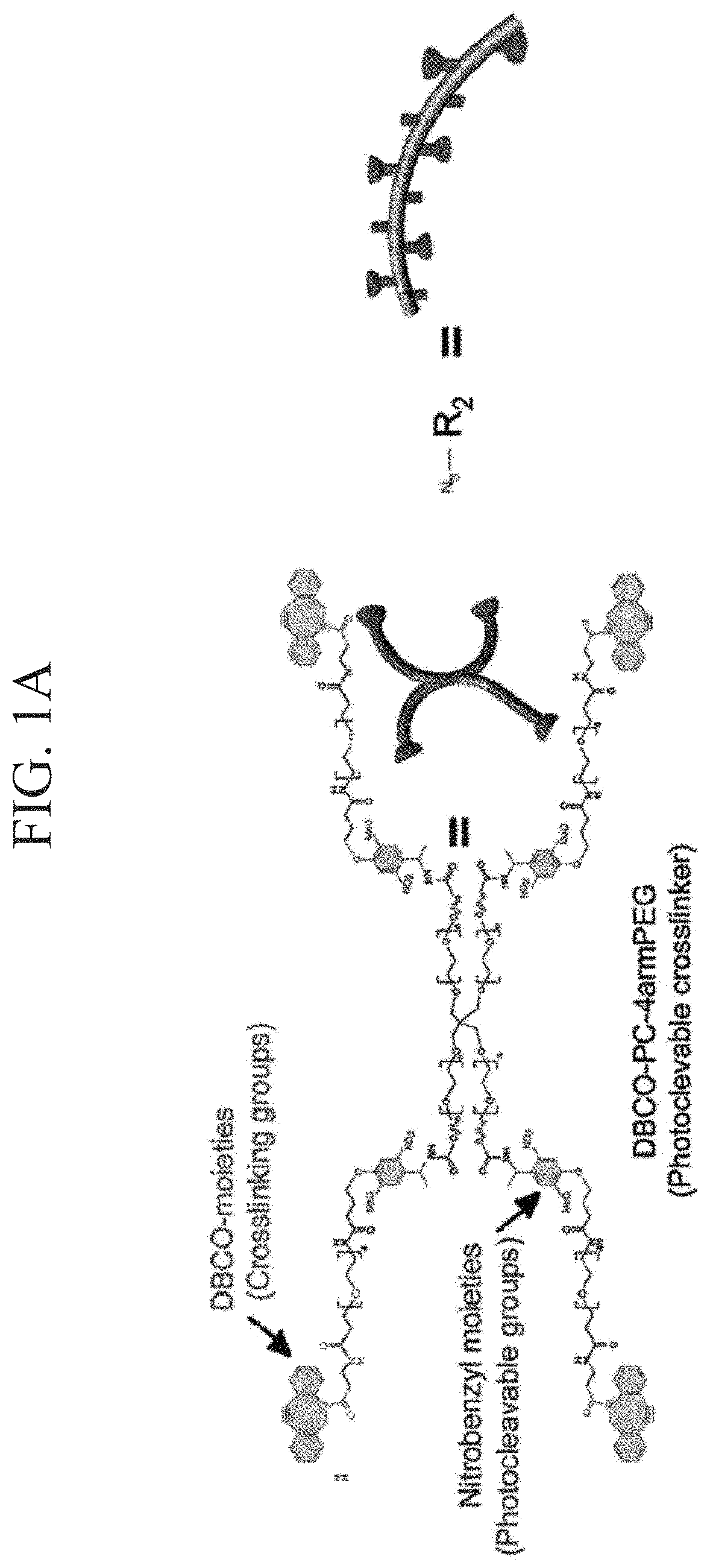
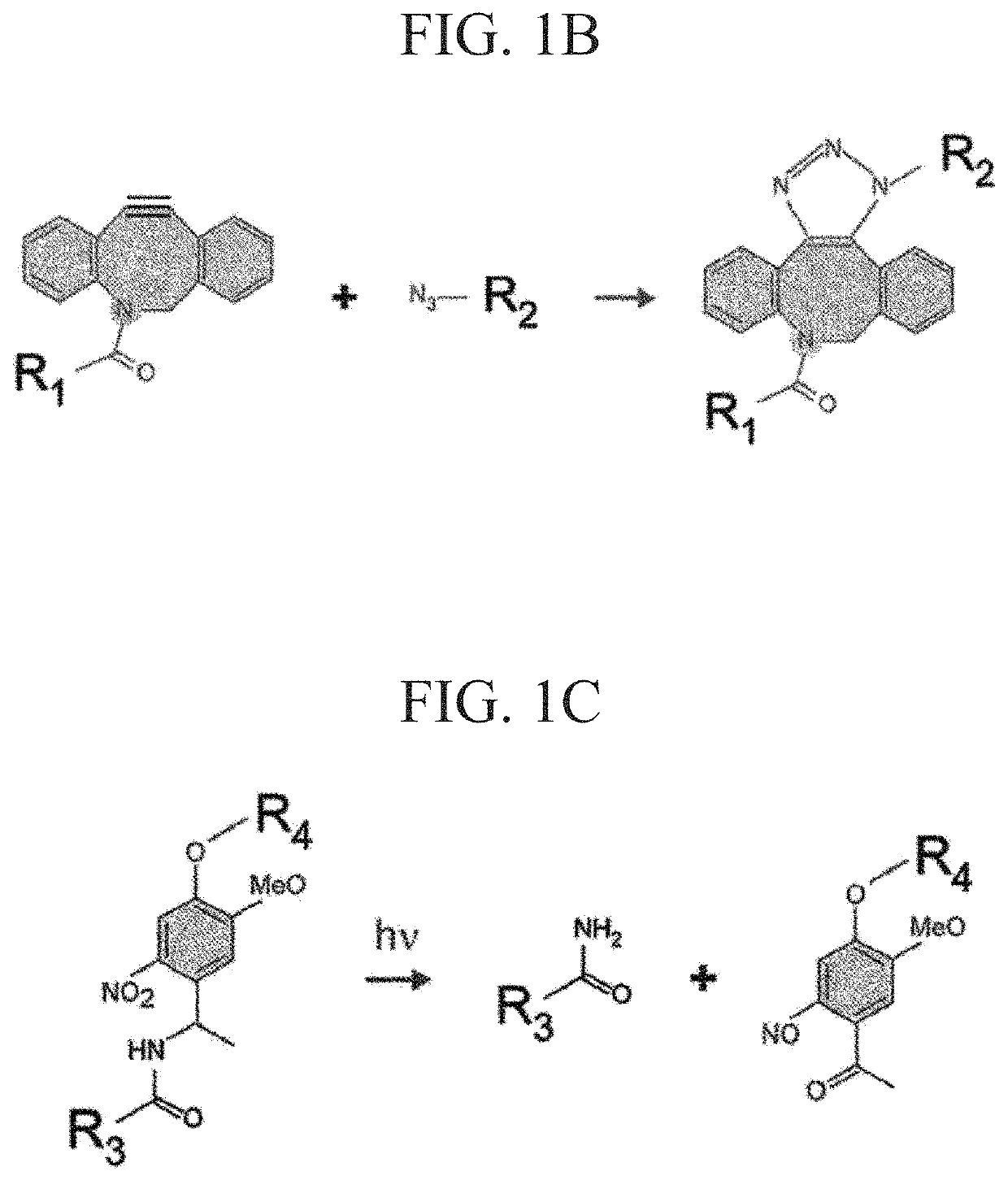
ActiveUS10774186B2Improve survivabilityLess cytotoxicityCell culture mediaCell culture supports/coatingPolyethylene glycolCross linker
Provided are a photodegradable hydrogel in which cells can be embedded in the photodegradable gel without causing cytotoxicity when the cells are embedded in the photodegradable gel by allowing the cells to coexist at the time of preparation of the photodegradable gel, and which contains a protein as one of the main components; a culture device using the same; a method for forming tissue; and a method for separating cells. A photodegradable hydrogel is obtained by condensation of an alkyne group contained in a cyclooctyne ring or an azacyclooctyne ring of the following compound A with an azido group of the following compound B. (Compound A) A compound is a photocleavable crosslinker which contains a main chain having a linear type- or a branched type- (of three or more branches) polyethylene glycol structure, a photodegradable nitrobenzyl group disposed at both terminals or a branched terminal of the main chain, and a group having a cyclooctyne ring or an azacyclooctyne ring disposed at a terminal side of the nitrobenzyl group. (Compound B) A compound is an azide-modified protein in which a main chain is a protein and at least some of an amino group present at lysine and arginine side chains of the main chain and an amino group present at a terminal of the main chain are modified with the azido group.
Owner:NAT INST OF ADVANCED IND SCI & TECH
A method for detecting the content of 3-amino-2-piperidone in compound amino acid injection
ActiveCN111337620BQuick balanceStrong specificityComponent separationAmino Acid InjectionIon-exchange resin
The invention relates to a method for detecting the content of 3-amino-2-piperidone, in particular to a method for detecting the content of 3-amino-2-piperidone in compound amino acid injection. The present invention utilizes high-performance liquid chromatography to detect the content of 3-amino-2-piperidone in the compound amino acid injection, and the chromatographic conditions overcome the weak retention of the C18 chromatographic column, the difficult balance of the amino column and the HILIC chromatographic column, and the ion-pair chromatography It has the advantages of fast column equilibration, excellent specificity, high sensitivity, good accuracy, good reproducibility, low limit of quantitation, etc., and the method is simple to operate and fast in analysis. It uses a strong cation exchange column Compared with the traditional method of manually processing samples with cation exchange resins, it is simpler and faster to directly separate and analyze the diluted samples online. The detection method of the present invention can be used for qualitative or quantitative analysis of 3-amino-2-piperidone in other amino acid products or raw materials such as ornithine hydrochloride and arginine.
Owner:费森尤斯卡比华瑞制药有限公司
Preparation method of environment-friendly high-temperature leveling agent for fabric
ActiveCN112726232ARaw material environmental protectionImprove production conditionsDyeing processEthylene oxideProcess engineering
The invention belongs to the technical field of fine chemicals, and particularly relates to a preparation method of an environment-friendly high-temperature leveling agent for fabric. Arginine and ethylene oxide are polymerized to prepare leveling agent mother liquor for the first time, and then a final product, the environment-friendly high-temperature leveling agent for the fabric, is prepared from the leveling agent mother liquor, urea and sulfamic acid through a one-pot method. Raw materials are green and environmentally friendly, production conditions are simpler and more convenient, and production is facilitated. The prepared environment-friendly high-temperature leveling agent for the fabric is green and environmentally friendly, and is excellent in transfer dyeing performance (the transfer dyeing rate is as high as 42.6%).
Owner:江苏文理新材料科技有限公司
Cellobiohydrolase mutant and its production method and application
ActiveCN109337888BReduce dosageImprove hydrolysis effectFungiMicroorganism based processesCelluloseThreonine
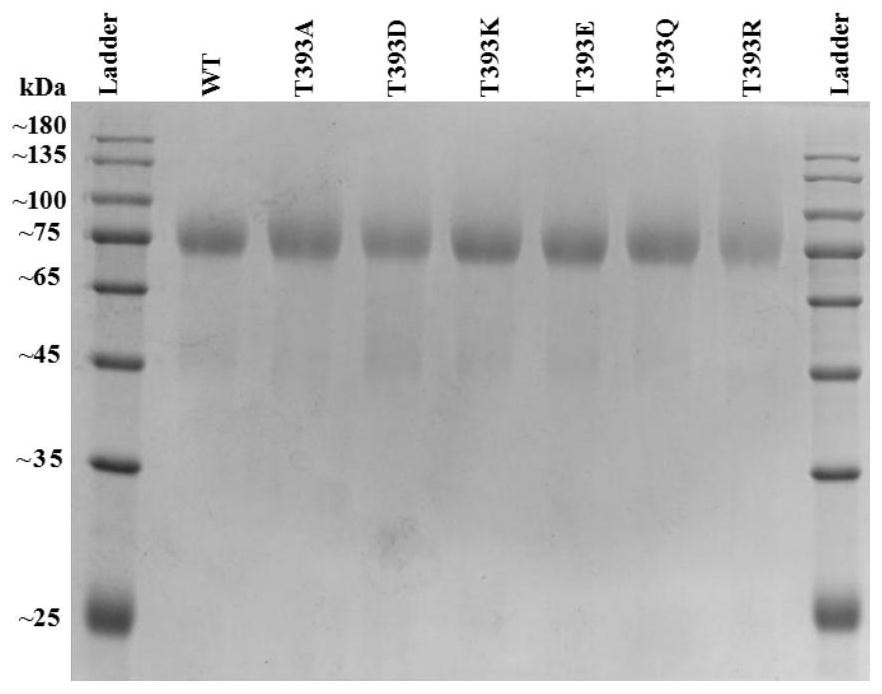
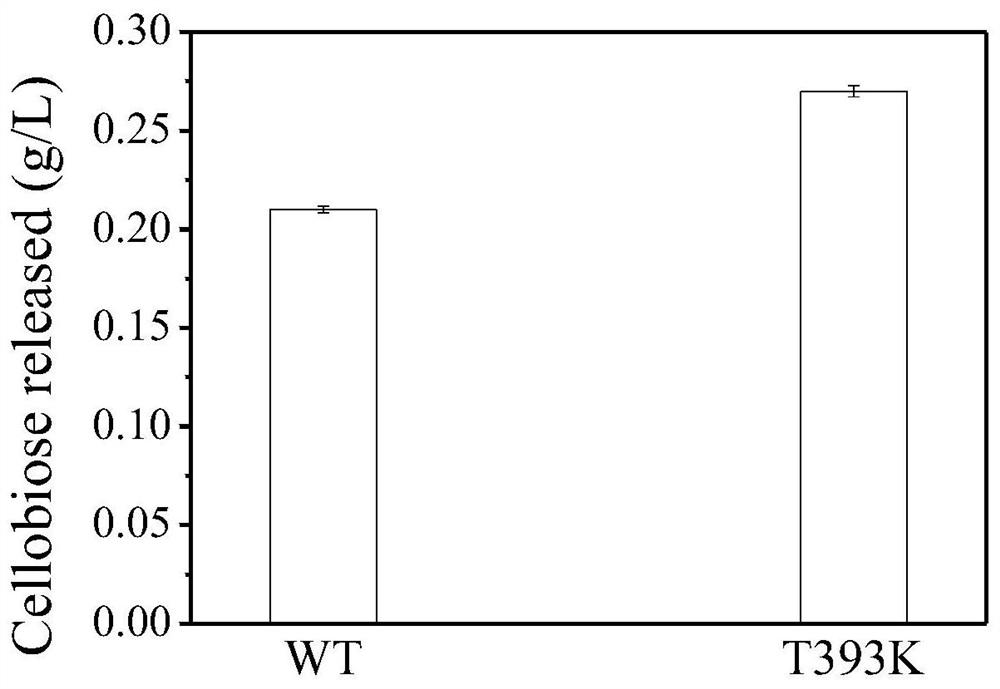
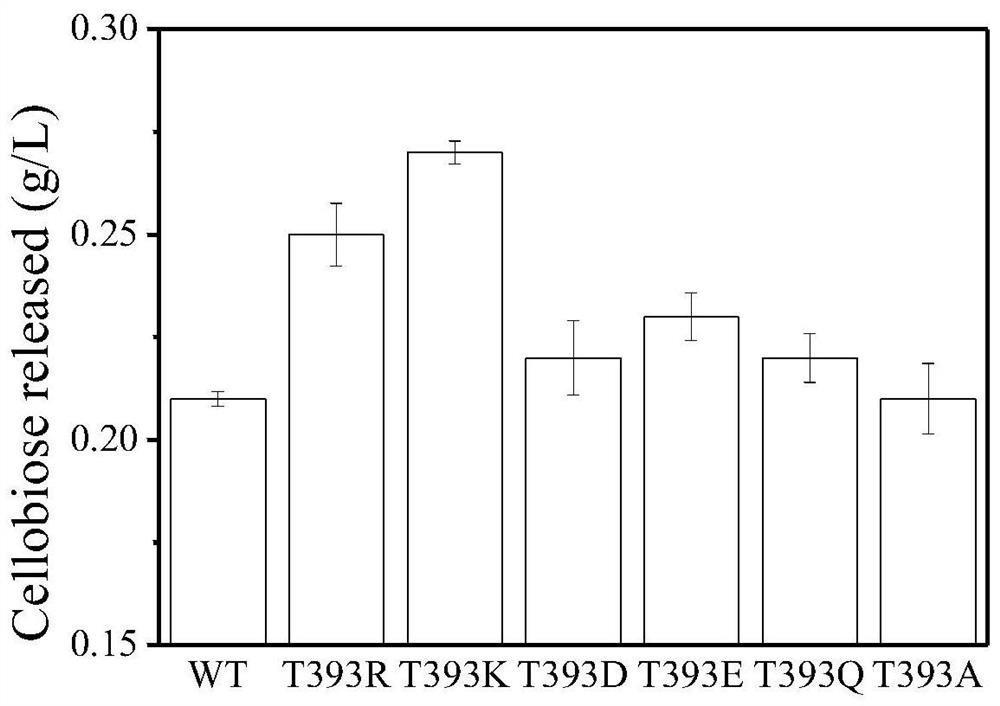
The present invention discloses a cellobiohydrolase mutant, whose activity provides the degradation function in cellulose degradation, and the enzyme is the following (a) or (b) protein: (a) as shown in SEQ ID NO: 1 Threonine at position 393 of the amino acid sequence is replaced by other amino acids, the other amino acids are lysine, arginine or glutamic acid, (b) the amino acid sequence in (a) has been substituted, deleted or added or a protein derived from (a) of several amino acids and having cellobiohydrolase activity. The invention also discloses a composition containing one or several enzymes. The invention also discloses the application of cellobiohydrolase mutant or composition in cellulose hydrolysis. The cellobiohydrolase mutant of the present invention has the activity of hydrolyzing cellulose, and a mutant with improved activity is obtained, and the effect of hydrolyzing cellulose is improved.
Owner:NANKAI UNIV
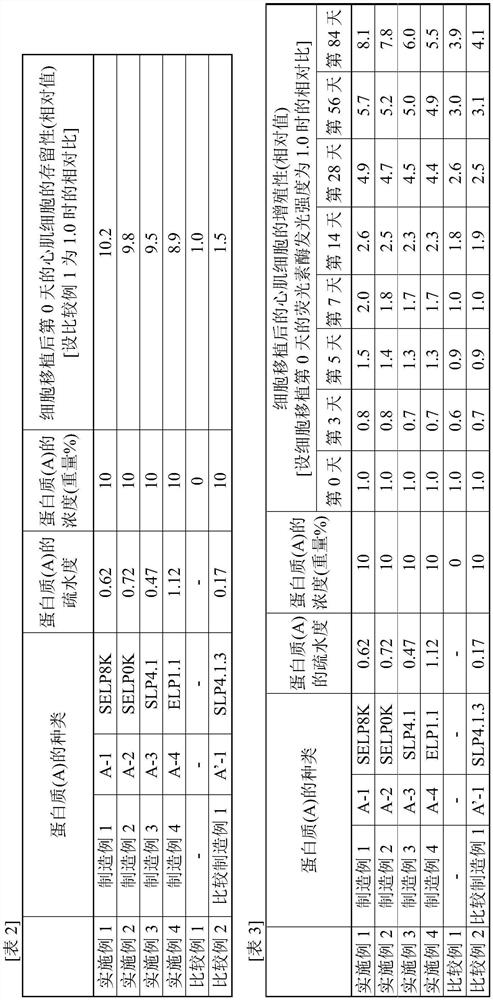
Composition for cell transplant, and method for cell transplant
PendingCN112292157AImprove retention capacityImprove proliferative abilityPeptide/protein ingredientsAntipyreticPost transplantProtein
Provided are a composition for cell transplant and a method for cell transplant that allow myocytes and / or cardiac progenitor cells to be suitably maintained in myocardial tissue and allow the survival and proliferation of transplanted cells to be improved. This composition for cell transplant comprises cells and an aqueous solution of a protein (A), and is characterized in that: the cells are myocardial cells and / or cardiac progenitor cells; protein (A) has a hydrophobicity of 0.2-1.2; the protein (A) has a polypeptide chain (Y) and / or a polypeptide chain (Y'); the total number of polypeptidechains (Y) and polypeptide chains (Y') in the protein (A) is 1-100; the polypeptide chain (Y) is an amino acid sequence (X) repeated 2-100 times, the amino acid sequence being any one of a VPGVG sequence (1) which is an amino acid sequence represented by SEQ ID NO:1, a GVGVP sequence (2) which is an amino acid sequence represented by SEQ ID NO:2, a GPP sequence, a GAP sequence, and a GAHGPAGPK sequence (3), which is an amino acid sequence represented by SEQ ID NO:3; the polypeptide chain (Y') is the polypeptide chain (Y) where 0.1-5% of the amino acid residues thereof are substituted with lysine residues and / or arginine residues; and the total number of the lysine residues and arginine residues is 1-100.
Owner:KYOTO UNIV +1
Composition for cell transplant, and method for cell transplant
ActiveUS20210252074A1Easy to keepIncreased persistencePeptide/protein ingredientsPharmaceutical delivery mechanismProteinArgininic acid
Provided are a composition for cell transplant and a method for cell transplant, both of which enable a myocardial tissue to favorably retain cardiac myocytes and / or cardiac progenitors and can improve the persistence and proliferation of transplanted cells. The composition for cell transplant of the present invention is a composition for cell transplant, containing cells and an aqueous solution containing a protein (A), the cells including a cardiac myocyte and / or a cardiac progenitor, the protein (A) having a degree of hydrophobicity of 0.2 to 1.2, the protein (A) containing a polypeptide chain (Y) and / or a polypeptide chain (Y′), the protein (A) containing 1 to 100 polypeptide chains as a total of the polypeptide chain (Y) and the polypeptide chain (Y′), the polypeptide chain (Y) being a polypeptide chain having 2 to 100 continuous amino acid sequences (X), the amino acid sequence (X) having any one of a VPGVG sequence (1) corresponding to an amino acid sequence of SEQ ID NO: 1, a GVGVP sequence (2) corresponding to an amino acid sequence of SEQ ID NO: 2, a GPP sequence, a GAP sequence, and a GAHGPAGPK sequence (3) corresponding to an amino acid sequence of SEQ ID NO: 3, the polypeptide chain (Y′) being a polypeptide chain having a structure in which 0.1 to 5% amino acid residues in the polypeptide chain (Y) are replaced by a lysine residue and / or an arginine residue and including 1 to 100 residues as a total of the lysine residue and the arginine residue.
Owner:KYOTO UNIV +1
Application of ethyl lauroyl arginate derivative as feed additive
ActiveCN108740356AChange solubilityChange stabilityOrganic active ingredientsClimate change adaptationOrganic acidChemistry
The invention relates to a novel feed additive, in particular to ethyl lauroyl arginate ion-pair compound derivative prepared by reaction of ethyl lauroyl arginate LAE and an organic acid. The derivative herein is used as a feed additive, can prevent and treat duckling Riemerella anatipestifer infection due to Riemerella anatipestifer, can treat peritonitis due to Escherichia coli and Staphylococcus aureus, can prevent and treat fish sepsis due to Aeromonas sobria, and has significantly better preventive and therapeutic effects than original LAE feed additives. The ethyl lauroyl arginate derivative prepared herein can degrade safely in human and animal bodies, has small toxic and side effects, can prevent occurrence of environmental drug-resistant bacteria due to emission of residual antibacterial agents to the environment, and has good antibacterial effect and low negative environmental influence.
Owner:EAST CHINA NORMAL UNIVERSITY
Recombinant corynebacterium crematum by expression of vitreoscilla haemoglobin gene and use thereof
InactiveCN101397548BImprove acid production performanceIncrease bacterial concentrationBacteriaMicroorganism based processesCorynebacterium crenatumGenetic engineering
Owner:GUANGDONG HUANXI BIOLOGICAL TECH
Electrocatalysis method for hydrolyzing esters and amide organic compound with high steric hindrance
The invention discloses a catalytic chemistry reaction method which is a technical method employing an alternating electric field to directly catalyze a hydrolysis reaction of ester and amide with high steric hindrance. The technical method is a hydrolysis reaction conducted in an electrolyzer, and a voltage of 20V peak and 1-100 Hz frequency is effected on a hydrolysis system of cedryl acetate, linalyl acetate and N-alpha-benzoyl-DL-arginine-4-nitroaniline. Substrate molecules are polarized in a Helmholtz layer to facilitate fractures of weak bonds, so as to catalyze the hydrolysis reaction without using a catalyst. The method is simply operated, at low costs and without catalyst pollution, and can be used for degradation of organics and pollutants with high steric hindrance.
Owner:NANKAI UNIV
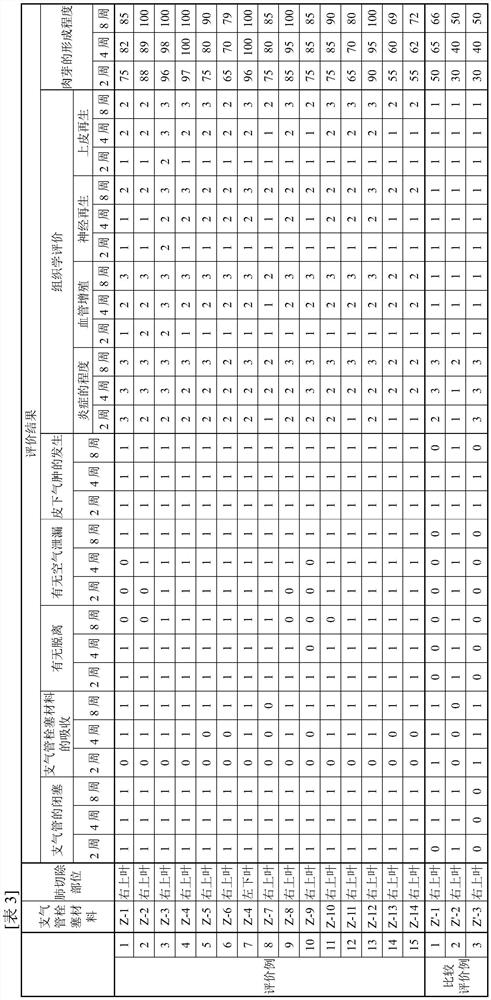
Bronchial embolic material
ActiveCN114746128APromote repairEasy to replaceSurgical adhesivesPeptide/protein ingredientsTissue repairTotal amino acids
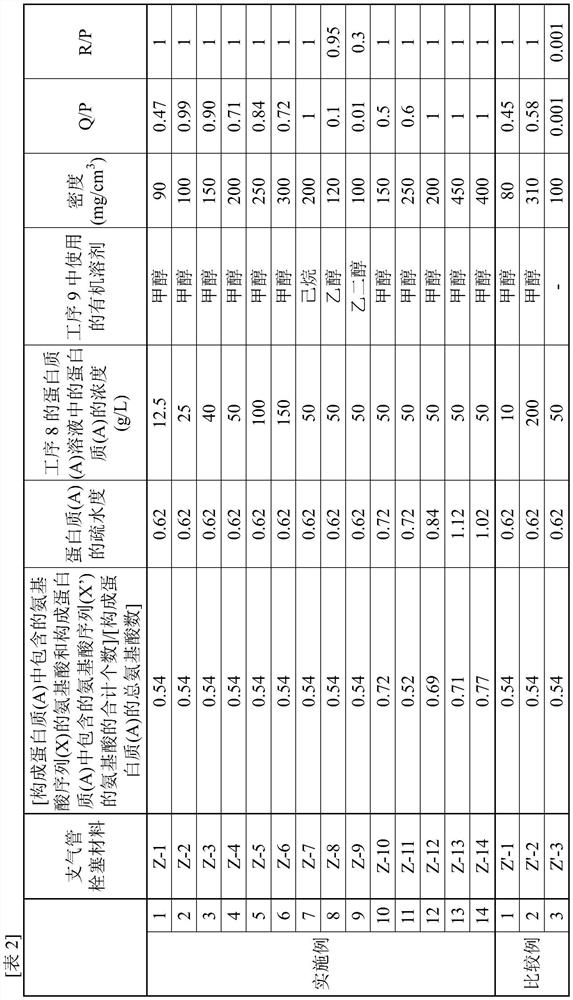
The present invention addresses the problem of providing a bronchial embolization material that enables tissue repair and replacement. This bronchial embolization material contains a protein (A), and is characterized in that: the protein (A) has a polypeptide chain (Y) and / or a polypeptide chain (Y '); the total number of the polypeptide chains (Y) and the polypeptide chains (Y ') in the protein (A) is 1-100; the polypeptide chain (Y) comprises 2-200 consecutive amino acid sequences (X) of at least one type of amino acid sequence (X) selected from the group consisting of a VPGVG sequence (1), a GVGVP sequence (4), a GPP sequence, a GAP sequence, and a GAHGPAGPK sequence (3), wherein the VPGVG sequence (1) is an amino acid sequence represented by SEQ ID NO: 1; the GVGVP sequence (4) is an amino acid sequence represented by SEQ ID NO: 4; the polypeptide chain (Y ') is a polypeptide chain in which 5% or less of the amino acids in the polypeptide chain (Y) are substituted with lysine and / or arginine, and the total number of lysine and arginine is 1-100; the protein (A) satisfies the following relational expression (1); the density of the bronchial embolism material is 90-450 mg / cm < 3 >; the bronchial embolization material satisfies the following relational expression (2). 0.50 < = [the total number of amino acids constituting the amino acid sequence (X) included in the protein (A) and amino acids constituting the amino acid sequence (X ') included in the protein (A)] / [the total number of amino acids constituting the protein (A)] < = 0.80 (1). The amino acid sequence (X ') is an amino acid sequence in which 60% or less of the amino acids in the amino acid sequence (X) are substituted with lysine and / or arginine. ] 0.01 < = Q / P (2) [In relational expression (2), P is the weight of a dried product (DP) obtained by drying the bronchial embolization material at 1 atmospheric pressure 100 DEG C for 3 hours and then drying at 1 atmospheric pressure 40 DEG C for 15 hours; q is the weight of a substance (DQ) obtained by immersing the dried product (DP) in water at 1,000 times the weight of P at 25 DEG C for 72 hours, then taking out the dried product (DP), drying the dried product (DP) at 100 DEG C for 3 hours, and then drying the dried product (DP) at 40 DEG C for 15 hours.
Owner:KYOTO UNIV +1
A kind of preparation method of magnetic resonance imaging contrast agent of 19f-acunotate nanoemulsion
ActiveCN110559456BSolve the problem of poor water solubilityGood biocompatibilityRadioactive preparation carriersRadioactive preparation formsPolyethylene glycolPorphyrin
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com