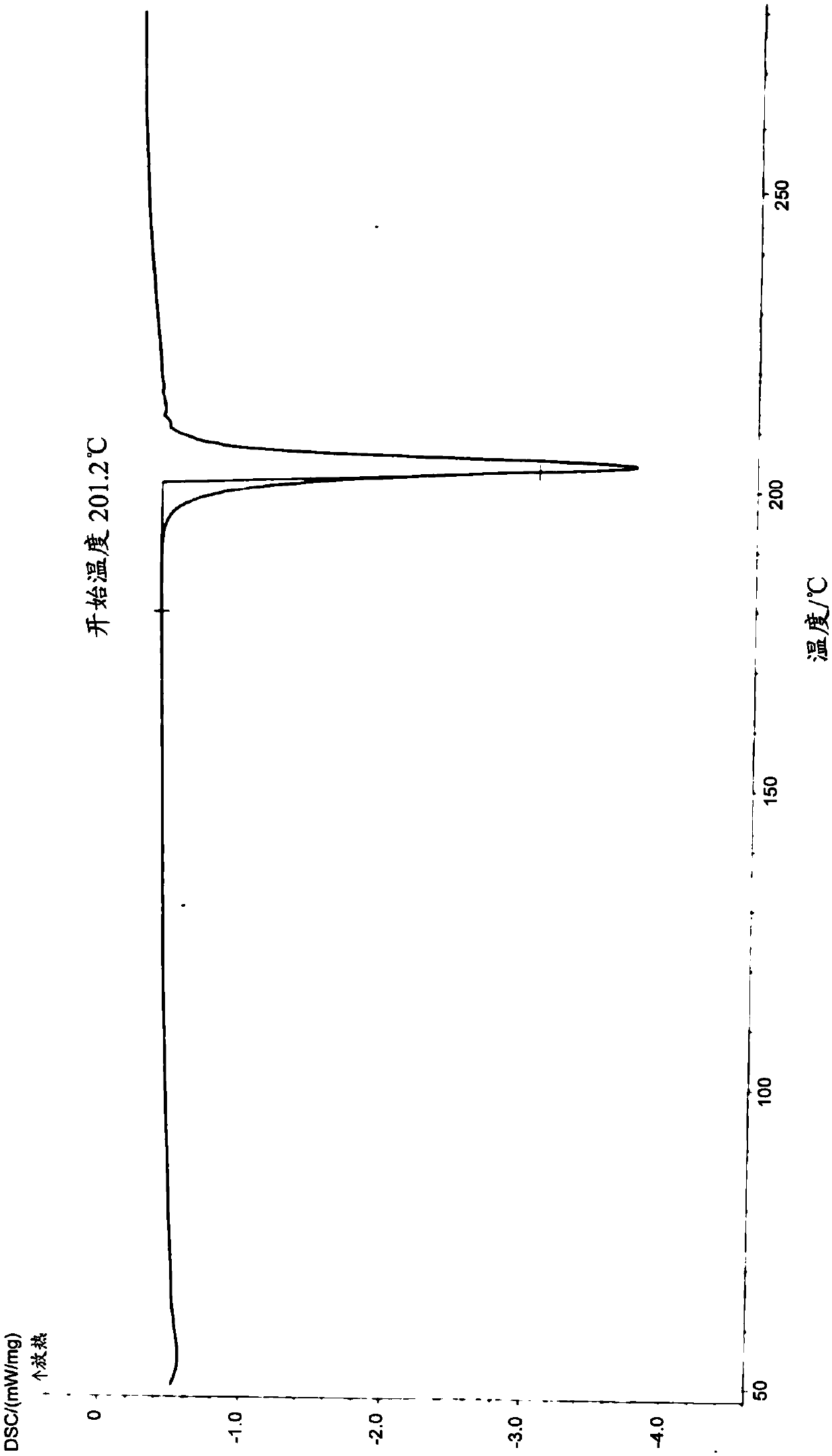
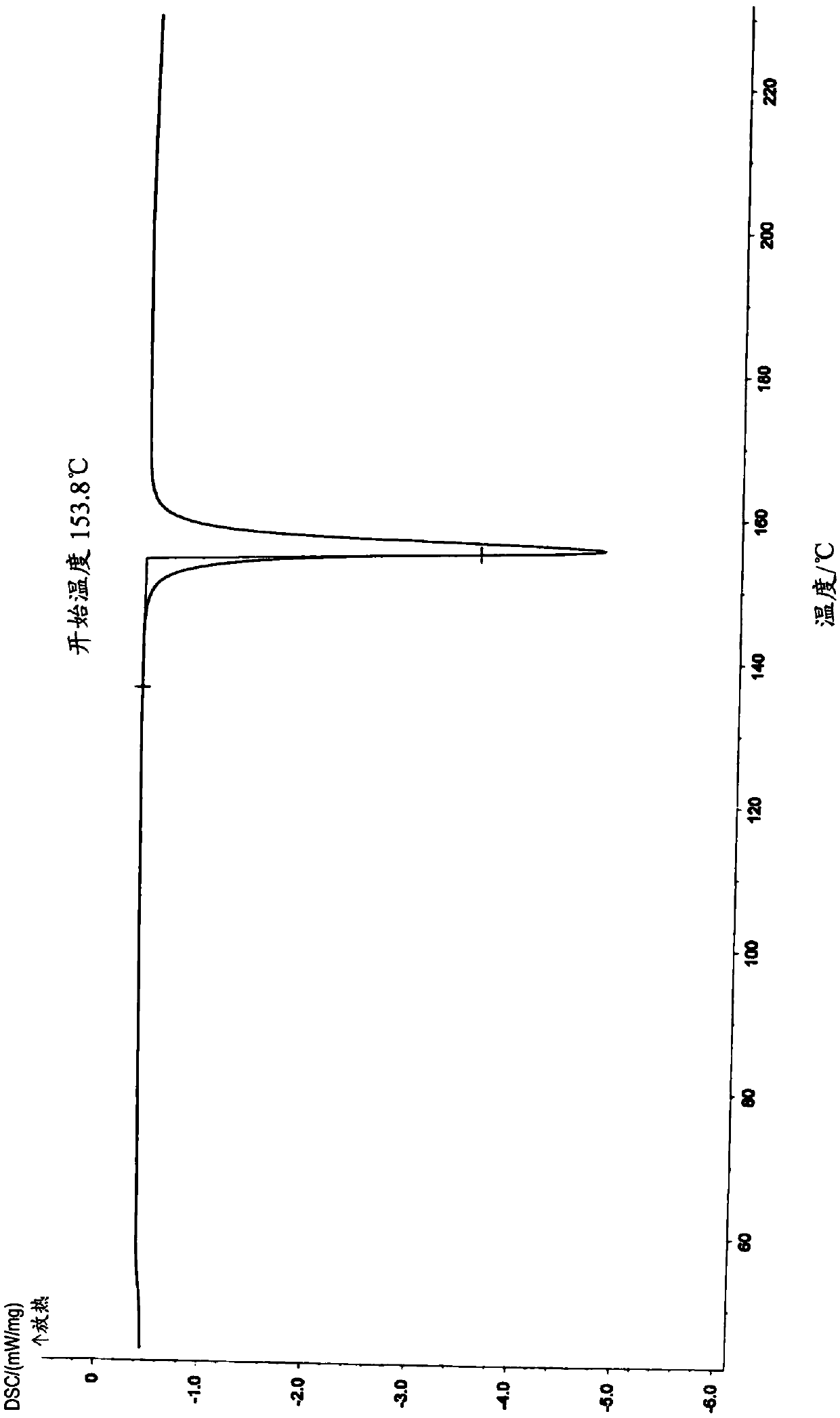
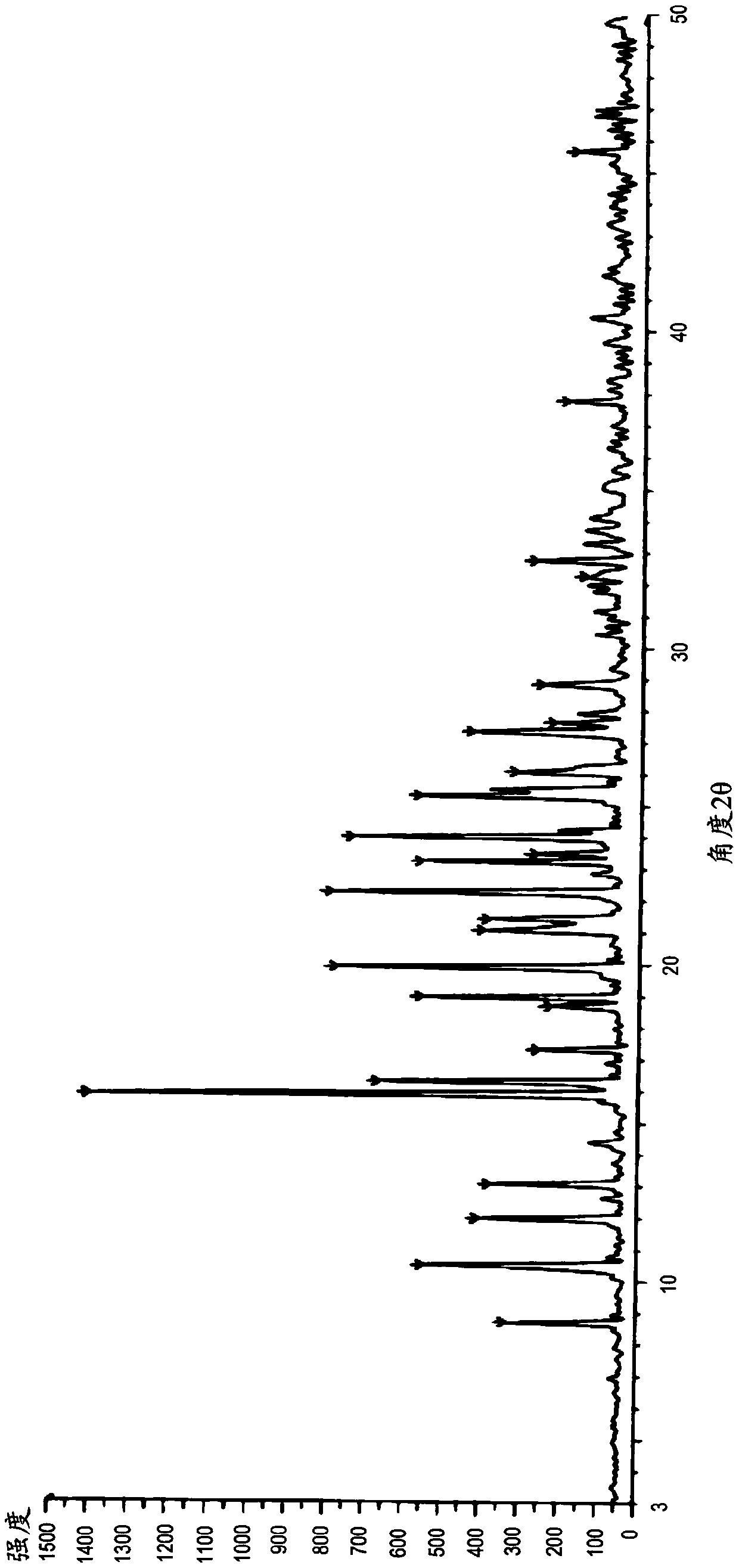
Pharmaceutical composition of benzodiazepine compound for nasal mucosa administration and preparation method and application thereof
A technology for mucosal drug delivery and compound, applied in the field of pharmaceutical preparations, can solve the problems of inconvenient medication for patients, high production cost, long production cycle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Compound (R) shown in embodiment 1 formula (I) 1 is methyl, R 2 Prepared for nasal mucosal administration of methyl) benzenesulfonate
[0070] The liposome prescription is as shown in the table,
[0071]
[0072] Preparation Process:
[0073] 1. Microparticle preparation:
[0074] (1) Compound (R) shown in formula (I) 1 is methyl, R 2 Be methyl) besylate as API and Soluplus, magnesium stearate micronization, mix homogeneously, after making physical mixture, add polyethylene glycol P6000.
[0075] (2) Set the extrusion temperature of the twin-screw extruder to 150±10°C, start the screw after rising to the set temperature, add the physical mixture in the step (1) to the extruder, heat-melt, extrude Press and extrude in the form of spherical particles to obtain amorphous particles, which are then micronized to obtain particles with a diameter of less than 150nm.
[0076] 2. Liposome preparation:
[0077] (1) Weigh the microparticles, lecithin, cholesterol, buffe...
Embodiment 2
[0083] Compound (R) shown in embodiment 2 formula (I) 1 is methyl, R 2 Preparation of pharmaceutical composition for ethyl) administration through nasal mucosa
[0084] The liposome prescription is as shown in the table,
[0085]
[0086] Preparation Process:
[0087] 1. Microparticle preparation:
[0088] (1) Compound (R) shown in formula (I) 1 is methyl, R 2 Be ethyl) as API and K30, magnesium stearate micronization, mix uniformly, after making physical mixture, add polyethylene glycol P6000.
[0089] (2) Set the extrusion temperature of the twin-screw extruder to 160±10°C, start the screw after rising to the set temperature, add the physical mixture in the step (1) to the extruder, heat-melt, extrude Press and extrude in the form of spherical particles to obtain amorphous particles, which are then micronized to obtain particles with a diameter of less than 150nm.
[0090] 2. Liposome preparation:
[0091] (1) Weigh the prescribed amount of microparticles, lecithi...
Embodiment 3
[0097] Compound (R) shown in embodiment 3 formula (I) 1 for hydrogen, R 2 Preparation of pharmaceutical composition for nasal mucosa administration
[0098] The liposome prescription is as shown in the table,
[0099]
[0100] Preparation Process:
[0101] 1. Microparticle preparation:
[0102] (1) Compound (R) shown in formula (I) 1 for hydrogen, R 2 For methyl) as API and K90, talcum powder micronization, mix well, after making physical mixture, add polyethylene glycol P2000.
[0103] (2) Set the extrusion temperature of the twin-screw extruder to 150°C, start the screw after rising to the set temperature, add the physical mixture in step (1) to the extruder, heat-melt and extrude, It is extruded in the form of spherical particles to obtain amorphous particles, and then micronized to obtain particles with a diameter of less than 150nm.
[0104] 2. Liposome preparation:
[0105] (1) Weigh microparticles, phosphatidylcholine, cholesterol, buffer, p-phenylenediamine,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com