Flame-retardant polyurea and synthesis method thereof
A synthetic method, flame-retardant technology, applied in the direction of polyurea/polyurethane adhesive, polyurea/polyurethane coating, adhesive type, etc., to achieve the effect of expanding application, simple and feasible synthesis method, excellent flame-retardant type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
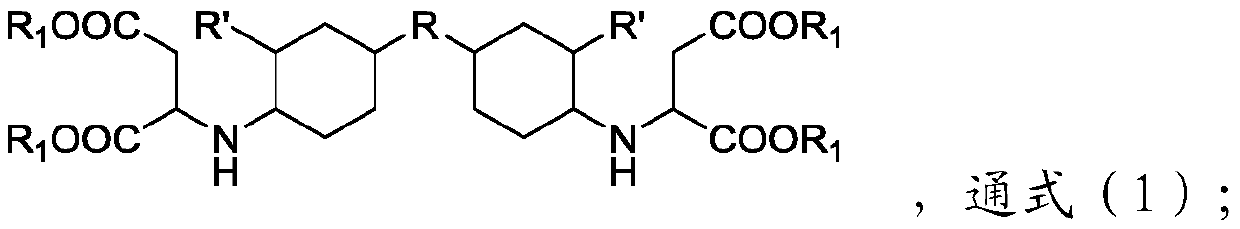
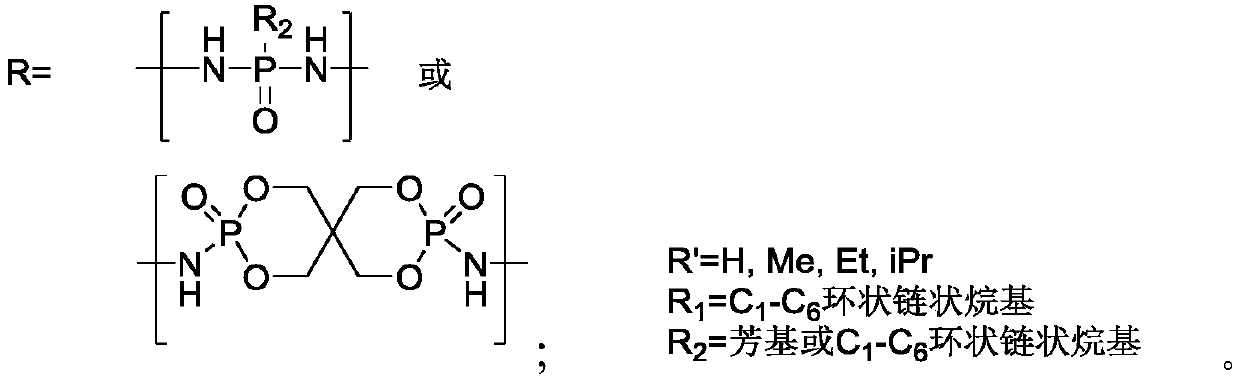
[0036] The flame retardant polyurea provided by the embodiment of the present invention has the following structure:
[0037]
[0038] The synthesis method is as follows:
[0039] first step:
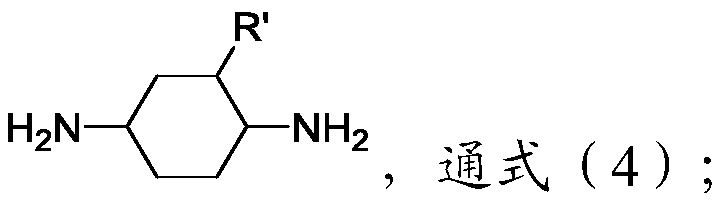
[0040] Weigh 1-methyl-2,4-cyclohexanediamine (2.56g, 0.02mol), phenylphosphonic dichloride (1.95g, 0.01mol), 1.5% 1-methyl-2 , 4-Cyclohexanediamine mass of AlCl 3 (0.03 grams);
[0041] Under an inert gas atmosphere, add a dropping funnel, a stirrer, anhydrous CaCl 2 Add 1-methyl-2,4-cyclohexanediamine, 3mL triethylamine and 20mL tetrahydrofuran into the condensing reflux device of the protective tube, and cool to 0°C in an ice-water bath; then add AlCl 3 , Dissolve phenylphosphonic dichloride in 10 mL of tetrahydrofuran, add dropwise within 1 hour, and react at 30°C for 24 hours to obtain phosphoramide.
[0042] Step two:
[0043] Under an inert gas atmosphere, the phosphonamide synthesized above was added to an excess of diethyl maleate (five equivalents), and the temperature...
Embodiment 2
[0045] The flame retardant polyurea provided by the embodiment of the present invention has the following structure:
[0046]
[0047] The difference between the preparation method of this example and Example 1 is that phenylphosphonic dichloride (1.95 g, 0.01 mol) is replaced by p-methoxyphenyl phosphonic dichloride (2.24 g, 0.01 mol).
Embodiment 3
[0049] The flame retardant polyurea provided by the embodiment of the present invention has the following structure:
[0050]
[0051] The difference between the preparation method of this example and Example 1 is that phenylphosphonic dichloride (1.95 g, 0.01 mol) is replaced by phenyl phosphonic dibromide (2.82 g, 0.01 mol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com