Method for analyzing sulfate ions in preparation process of lithium ion battery
A sulfate ion and lithium ion battery technology, which is applied in the direction of material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, can solve the problem of low accuracy and uncertainty of the specific value of the test results. , Organic solvents and water insoluble problems, to achieve the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
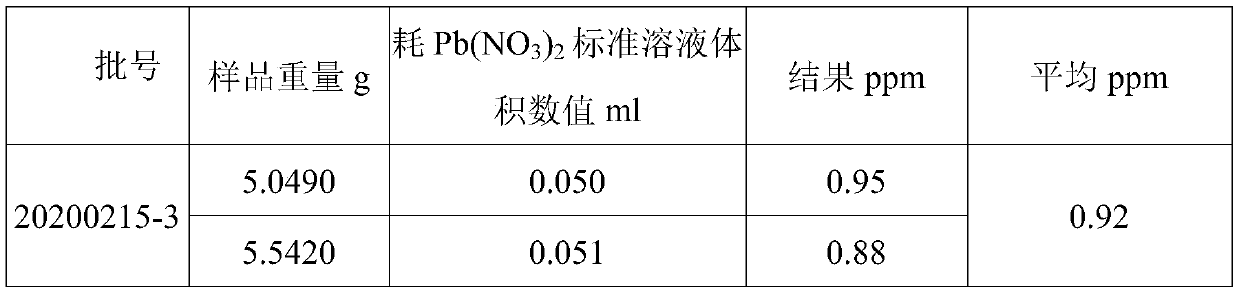
[0043] Lithium hexafluorophosphate (LiPF 6 ) detection of sulfate ion in the electrolyte, the electrolyte consists of 54.3wt% (wt%, mass percent) of dimethyl carbonate (DMC), 7.7wt% of diethyl carbonate (DEC), 27wt% % ethylene carbonate (EC), 10wt% LiPF 6 And the vinylene carbonate of 1wt% is formulated, comprises the following steps:
[0044] 1. Instruments and reagents
[0045] (1) microburette 2ML, division value 0.01ML;
[0046] (1) One ten-thousandth balance (accurate to 0.0002g);
[0047] (2) Nitric acid solution (8+92), analytically pure;
[0048] (3) Acetic acid (glacial acetic acid), analytically pure;
[0049] (4) nitric acid, AR grade; Ammonia (mass percentage concentration is 25%), analytically pure;
[0050] (5) 100ml glass beaker, 1 glass stirring rod;
[0051] (6) Nitric acid solution (1+2000), analytically pure;
[0052] (7) ethanol (mass percentage concentration is 95%), analytically pure;
[0053] (8) Hexamethylenetetramine, analytically pure;
[0054...
Embodiment 2
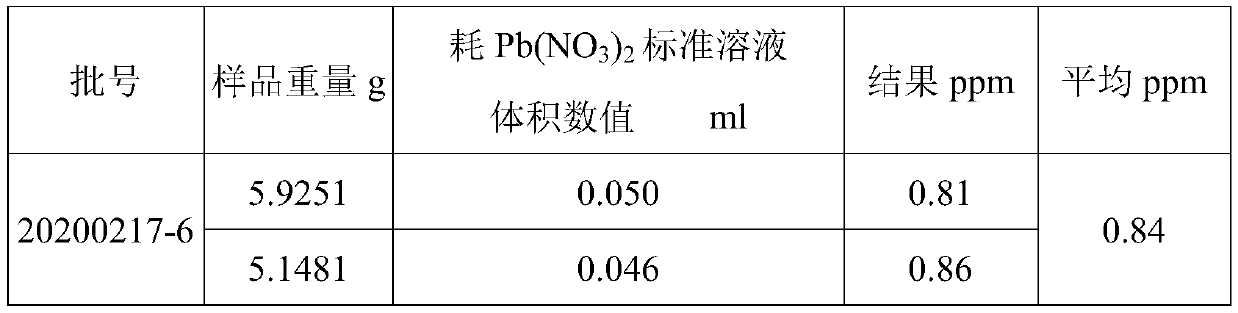
[0086] Electrolyte containing lithium difluorooxalate borate (LiODFB) (by 58wt% of DMC, 27wt% of EC, 10wt% of LiPF 6 , the LiODFB of 1.5wt% and the 1.3-propane sultone of 3.5wt% are formulated) the detection of sulfate ion in, comprises the following steps:
[0087] 1. Instruments and reagents
[0088] (1) microburette 2ML, division value 0.01ML;
[0089] (2) One ten-thousandth balance (accurate to 0.0002g)
[0090] (3) Nitric acid solution (8+92), analytically pure;
[0091] (4) Acetic acid (glacial acetic acid), analytically pure;
[0092] (5) Nitric acid, AR grade; Ammonia (mass percentage concentration is 25%), analytically pure;
[0093] (6) 100ml glass beaker, 1 glass stirring rod;
[0094] (7) Nitric acid solution (1+2000), analytically pure;
[0095] (8) ethanol (mass percentage concentration is 95%), analytically pure;
[0096] (9) Hexamethylenetetramine, analytically pure;
[0097] (10) Acetone, analytically pure;
[0098] (11) Disodium ethylenediaminetetraa...
Embodiment 3
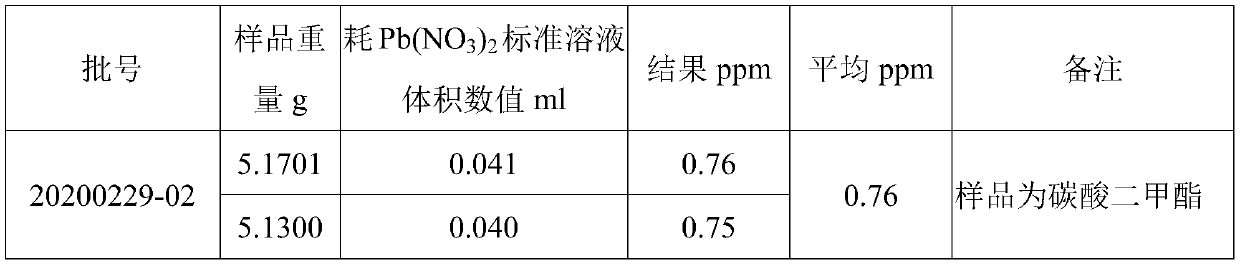
[0129] The detection of sulfate ion in solvent dimethyl carbonate comprises the following steps:
[0130] 1. Instruments and reagents
[0131] (1) microburette 2ML, division value 0.01ML;
[0132] (2) One-ten-thousandth balance (accurate to 0.0002g);
[0133] (3) Nitric acid solution (8+92), analytically pure;
[0134] (4) Acetic acid (glacial acetic acid), analytically pure;
[0135] (5) Nitric acid, AR grade; Ammonia (mass percent concentration is 25%), analytically pure
[0136] (6) 100ml glass beaker, 1 glass stirring rod;
[0137] (7) Nitric acid solution (1+2000), analytically pure;
[0138] (8) ethanol (mass percentage concentration is 95%), analytically pure;
[0139] (9) Hexamethylenetetramine, analytically pure;
[0140] (10) Acetone, analytically pure;
[0141] (11) Disodium ethylenediaminetetraacetic acid [c(EDTA)=0.05mol / L;
[0142] (12) Xylenol Orange (2g / L);
[0143] (13) Dithizone, analytically pure;
[0144] (14) Lead nitrate, analytically pure
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com