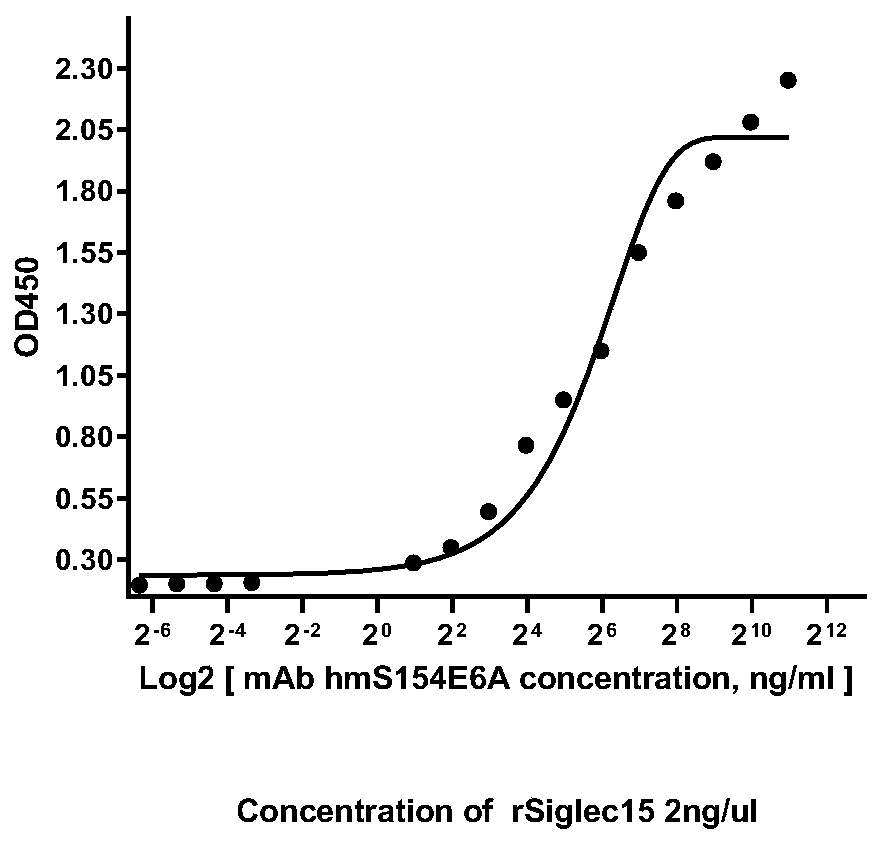
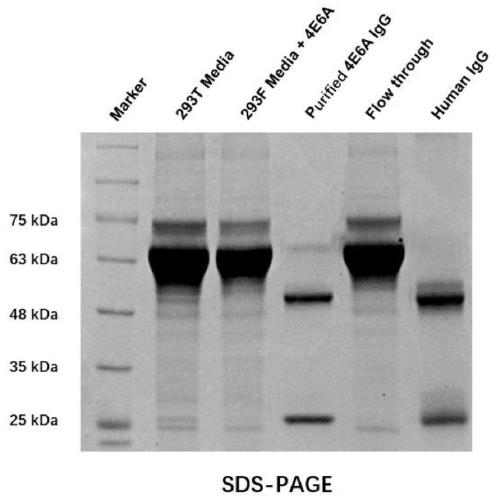
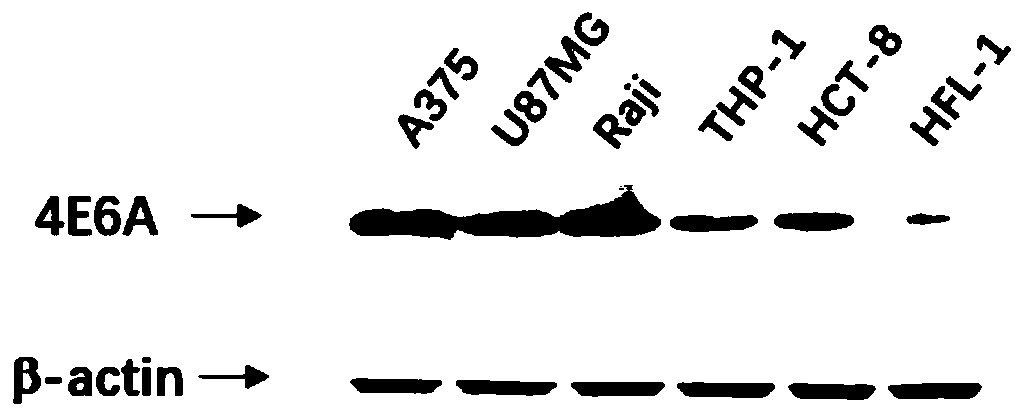
human-mouse chimeric Siglec-15-resistant neutralizing full-molecule IgG and preparation method and application thereof
A molecular, DNA molecular technology used in the field of biopharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Siglec-15 Extracellular Region Peptide Design
[0038] Siglec-15 has a total of 328 amino acids, and the extracellular region consists of an N-terminal V-set domain (A49-A165) including a sialic acid binding site and a type 2 constant region (IgC2) region (A168-A251), in which IgV The region is the key site for the combination of Siglec-15 and Sialyl-Tn. Using Siglec-15A37-A180 amino acids as a template, Overlap polypeptides were designed and named A1~A12 and B1~B11, respectively. After the peptides were synthesized, the fractions were mixed and coupled to OVA and KLH respectively.
[0039] A1 HSSPAQRWSMQV B1 RWSMQVPPEVSA A2 PPEVSAEAGDAA B2 EAGDAAVLPCTF A3 VLPCTFTHPHRH B3 THPHRHYDGPLT A4 YDGPLTAIWRAG B4 AIWRAGEPYAGP A5 EPYAGPQVFRCA B5 QVFRCAAARGSE A6 AARGSELCQTAL B6 LCQTALSLHGRF A7 SLHGRFRLLGNP B7 RLLGNPRRNDLS A8 RRNDLSLRVERL B8 LRVER LAL ADDR A9 ALADDRRY FCRV B9 RYFCRVEFAG...
Embodiment 2
[0040] Example 2: Preparation of mouse-derived anti-Siglec-15 hybridoma cells
[0041] Customize the recombinant Siglec-15 whole gene protein according to the human Siglec-15 gene. The protein and the mixed polypeptide coupled with OVA were used as immunogens to immunize the abdomen of pure BALB / c mice by subcutaneous injection, 100 μg / ml each time, five times in total, of which recombinant Siglec- 15 whole gene proteins, the 2nd and 4th use the mixed polypeptide coupled with OVA. When positive serum with an OD greater than 2 can be detected in the peripheral serum, the cells are fused, and the mixed peptide coupled with OVA is used to boost immunization 3 days before the fusion. On the day of fusion, the mouse spleen was taken, and a single-cell suspension was prepared with DMEM medium (GIBCO, USA). In the presence of 50% PEG (PH 8.0), the spleen cells and SP2 / 0 mouse myeloma cells were fused, and used HAT selective medium (DMEM medium 98ml, HT stock solution 1ml, A stock s...
Embodiment 3
[0042] Example 3: Screening, strain determination and identification of murine anti-Siglec-15 antibodies
[0043] The enzyme-linked immunoassay (ELISA) detection and screening was carried out according to the growth condition of the hybridoma cells, and the specific method is as follows. The recombinant Siglec-15 protein was used to coat the plate, and the cells in the positive wells were cloned and cultured again. After three times of subcloning, the positive clones were determined. The plates were then coated with Siglec-15 mixed polypeptides coupled with KLH to detect positive clones. After all the monoclonal cells in the wells were positive for Siglec-15-KLH and negative for KLH, several wells were taken for expansion and cultured and partially frozen.
[0044] ELISA method to screen Siglec-15 positive but normal mouse seronegative monoclonal antibody, the specific method is as follows:
[0045] (1) The chemically synthesized Siglec-15-KLH polypeptide was coated with ELI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com