T cell receptors recognizing mutated p53
A cell-receptor, specific technology for the treatment or prevention of cancer in mammals to address the limited options for cancer treatment, the unmet need for additional cancer treatments, and poor cancer prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] This example demonstrates the isolation and specific reactivity of 4 TCRs against mutated p53 from patient 4127.
[0168] combine Figure 1-7 Experiments were performed on patient 4127 as described in , 36 and 37A-37C. Mutant p53-reactive T cells in this patient were identified by the methods described in US Patent Application No. 2017 / 0224800 ("Tran Method"). The method for isolating individual TCRs is described below.
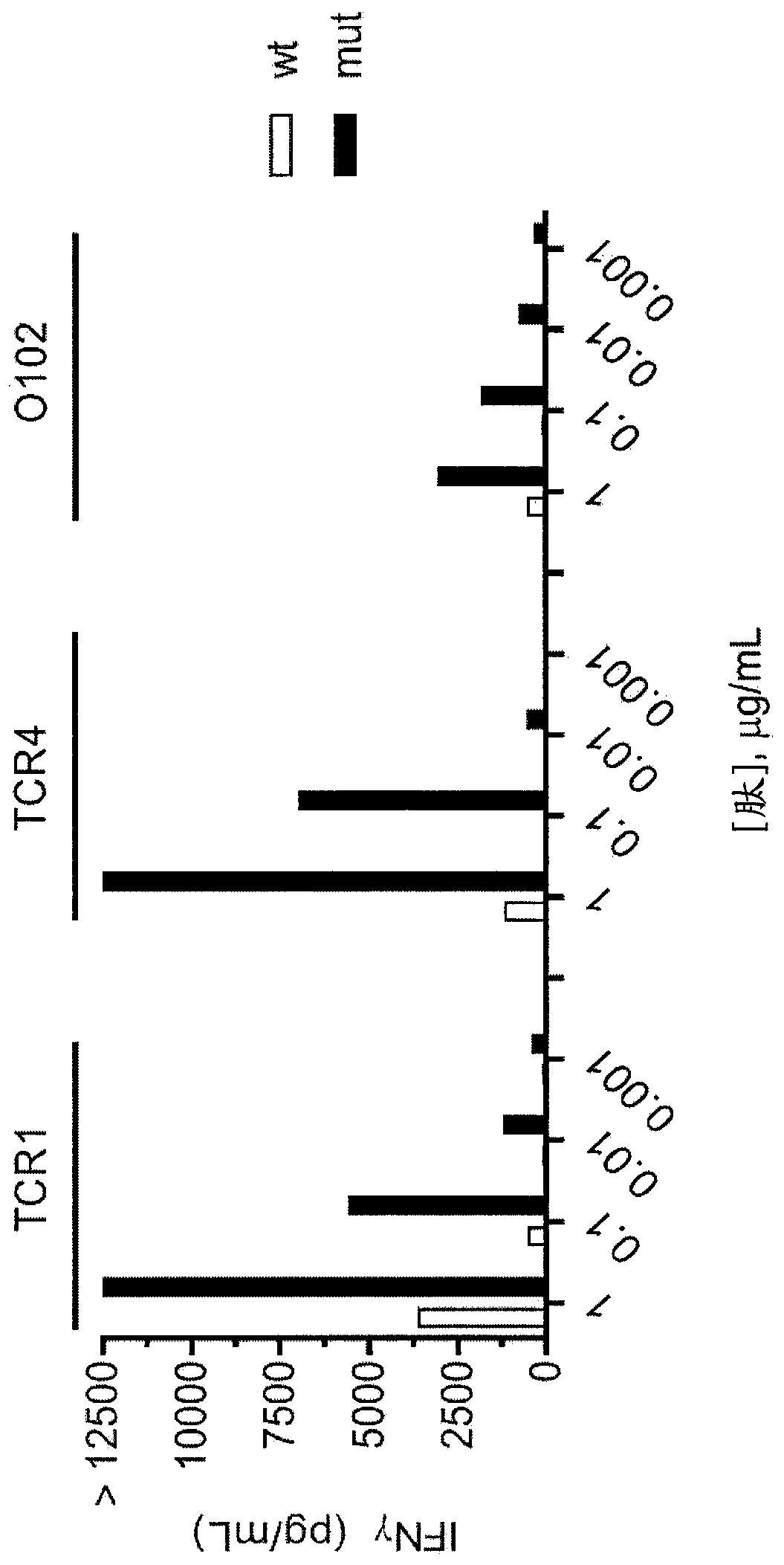
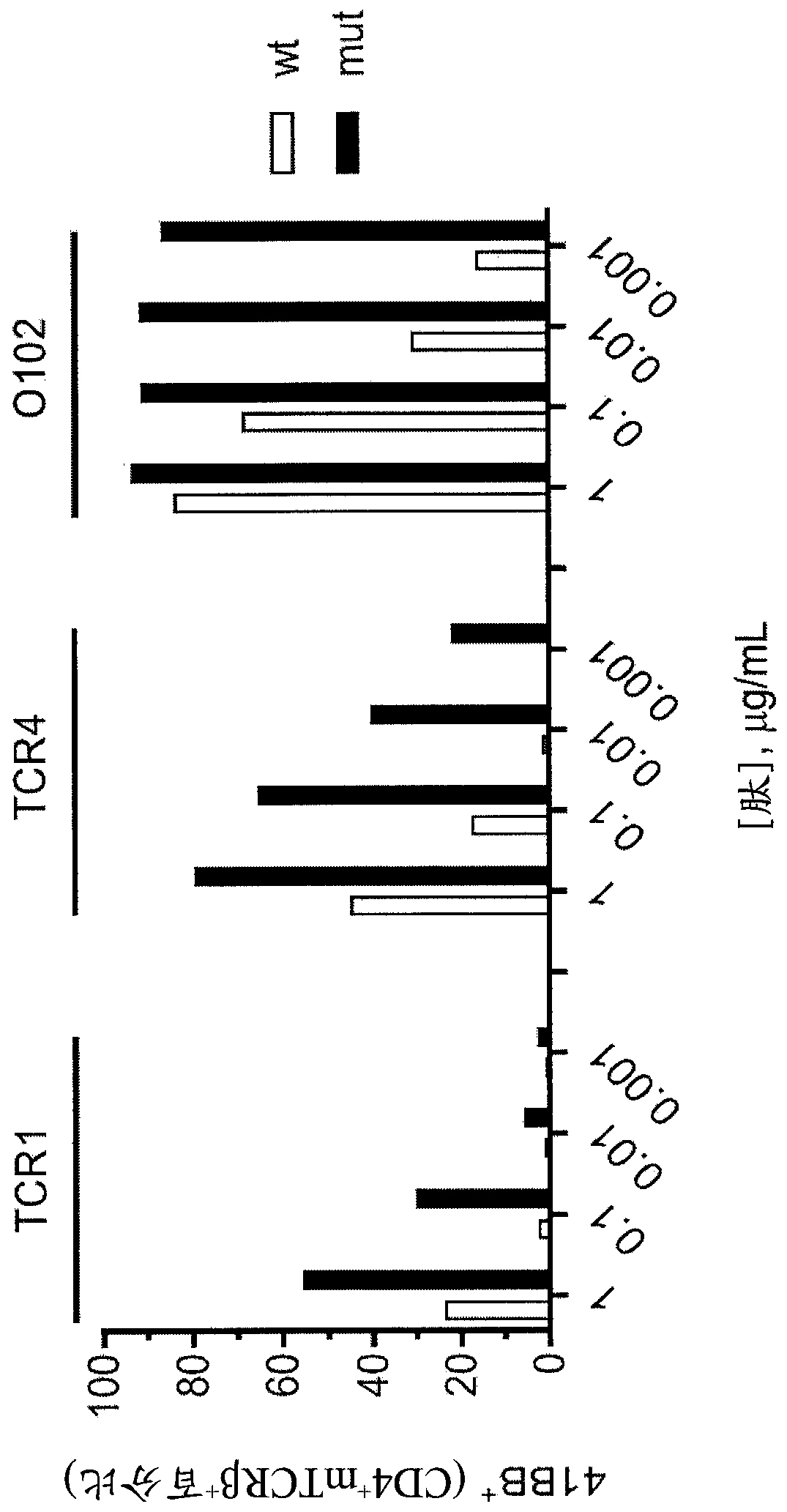
[0169] use figure 1 One of the TCRs shown in transduced autologous PBLs and tested 2 weeks later. Autologous DC cells were plated at 3 x 10 4 Cells / well were plated and pulsed overnight with decreasing concentrations of WT p53-G245 peptide (wt) or mutant p53-G245S peptide (mut). Add 3x 10 per hole 4 Transduced T cells were co-cultured overnight at 37°C. The supernatant was harvested for IFN-γ ELISA. IFN-γELISA results see figure 1 . 4-1BB expression by FACS gating: lymphocytes\PI(neg)CD3 + \CD3 + MTCR + \CD8(neg)CD4 + . For FACS results ...
Embodiment 2
[0233]This example demonstrates the isolation and specific reactivity of 3 anti-mutant p53 TCRs from patient 4196.
[0234] Experiments were performed on Patient 4196 as described in Figures 8-11. The patient's p53-reactive T cells were identified by the Tran method as described in US Patent Application No. 2017 / 0224800. Individual TCRs were isolated using the Fluidigm method. The statistics of patient 4196 are shown in Table D.
[0235] Characterization of p53-responsive cells: the challenge of identifying the minimal epitope: The first predicted peptide with the mutated amino acid p53 R 175H is shown in Table C.
[0236] Form C
[0237] HLA HLA-A0201 Peptide HMTEVVRHC (SEQ ID NO: 530) core HMTEVVRHC (SEQ ID NO: 530) Affinity (nM) 7826.06 %grade 13.00
[0238] MG1 vs 4196-Rx1 TIL:COS7 cells were transfected with plasmids for TMG1 and HLA alleles. Cells were co-cultured with 4196Rx1 TIL infusion bags for 20 hours (h). see the res...
Embodiment 3
[0270] This example demonstrates the isolation of 11 anti-mutant p53 TCRs from patient 4238.
[0271] as for Figure 12-15 The experiment was performed on patient 4238 as described.
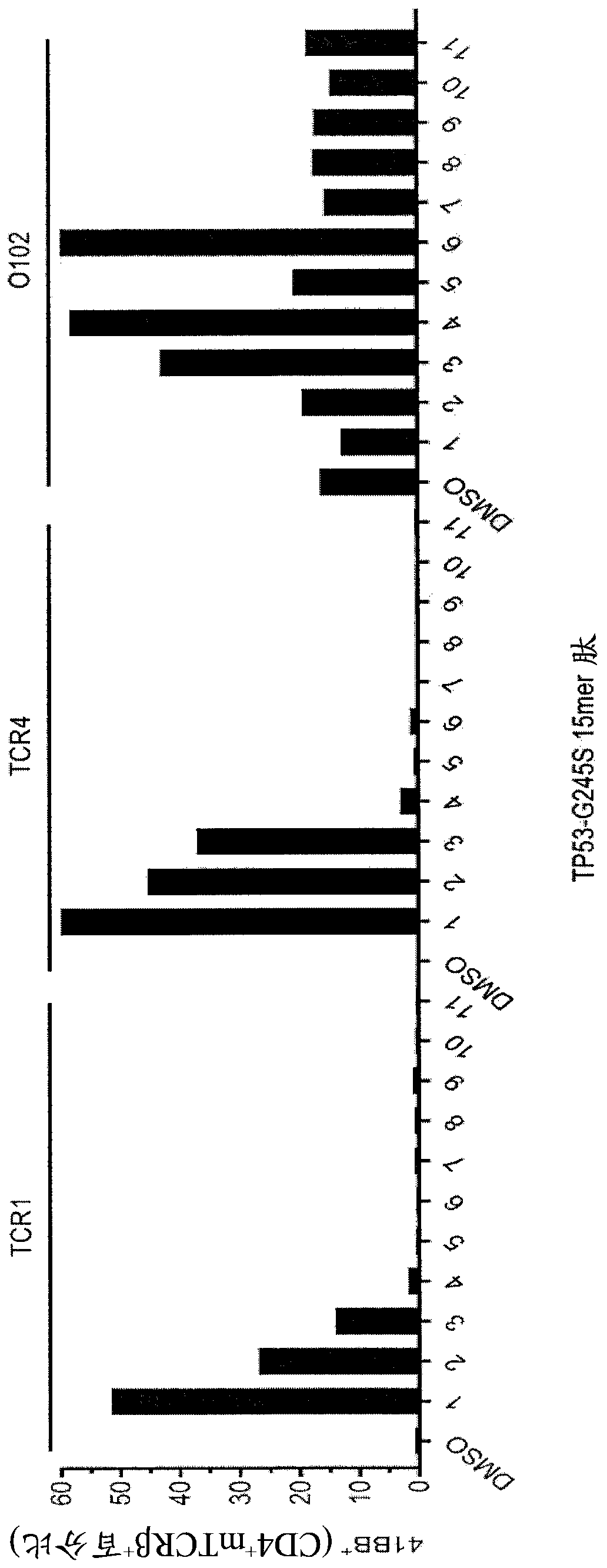
[0272] TIL fragments from patient 4238 (F1-F24, n=24) were co-cultured with TMG electroporated autologous APCs consisting of unrelated or mutated p53 sequences. Co-cultures were grown overnight at 37°C. IFN-γ secretion was assessed using the ELISPOT assay. see the results Figure 12 . Expression of 4-1BB was assessed by flow cytometry after gating on lymphocytes→live cells (PI negative)→CD3+ (T cells). see the results Figure 14 .
[0273]TIL fragments of patient 4238 (F1-F24, n=24) were co-cultured with autologous APCs pulsed with peptide vehicle (DMSO) or with a purified 25 amino acid peptide consisting of the mutant p53-R248Q sequence (HPLC method >95%) pulsed. Co-cultures were grown overnight at 37°C. IFN-γ secretion was assessed using the ELISPOT assay. see the results Figure 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com