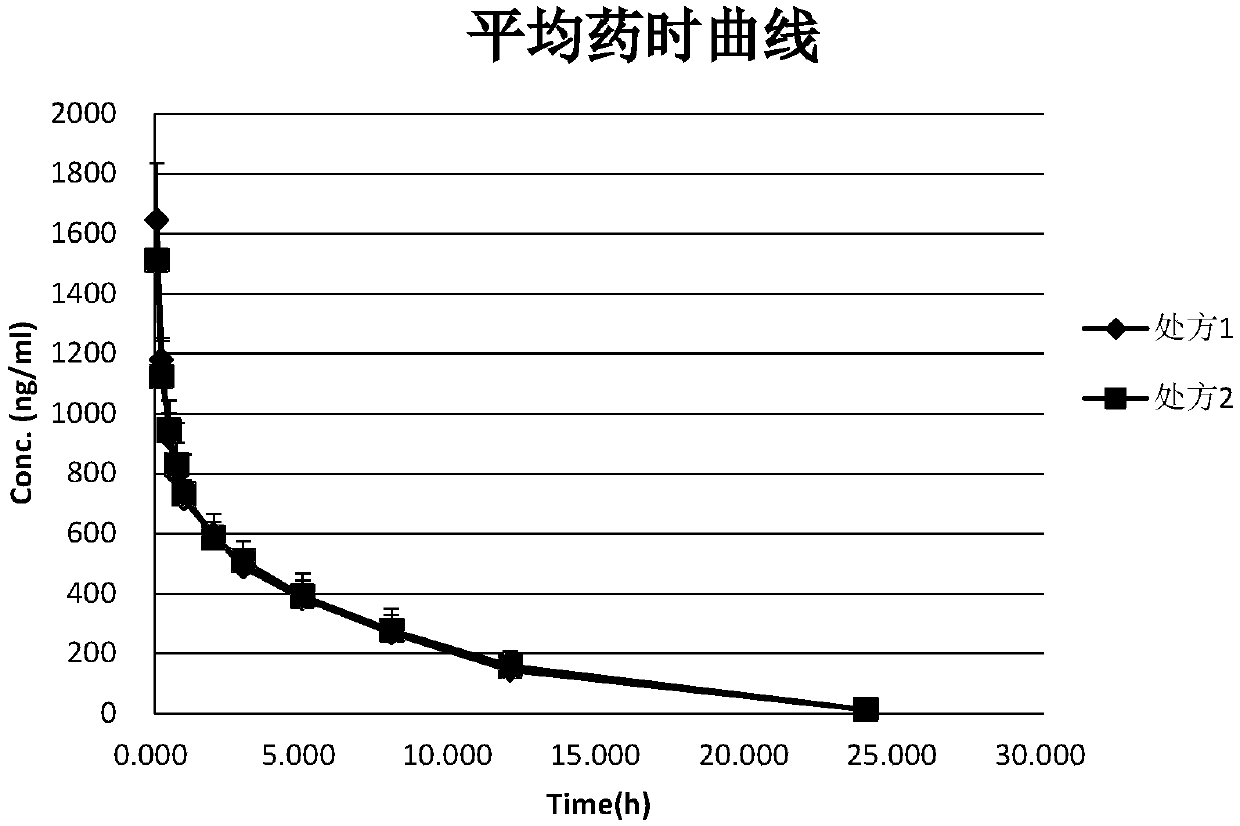
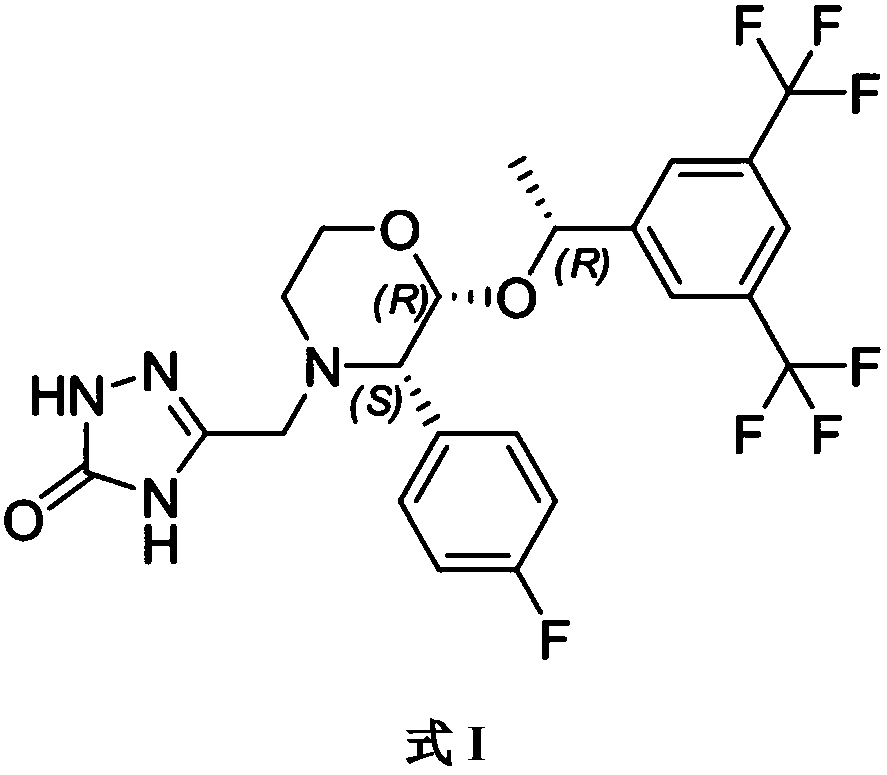
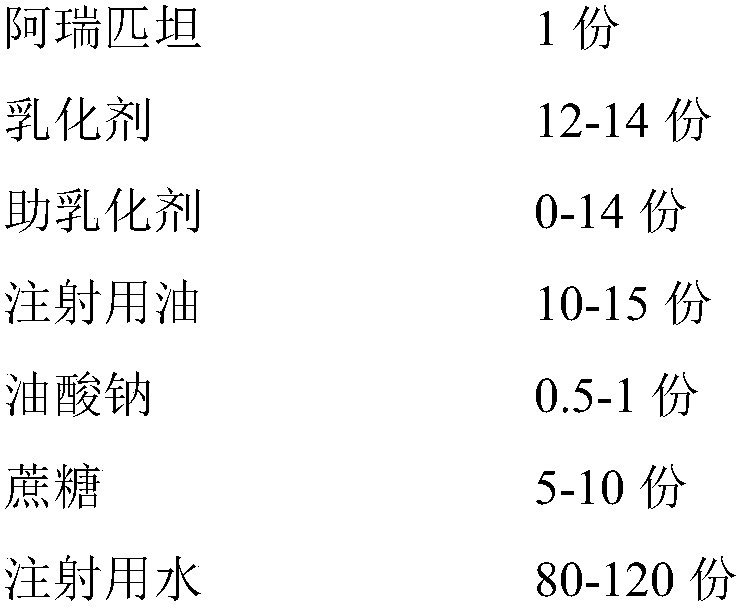
Aprepitant emulsion
A kind of aprepitant emulsion, emulsion technology, applied in emulsion delivery, drug combination, organic active ingredients and other directions, can solve problems such as increased technical requirements, increased emulsion viscosity, increased technological difficulty, etc., to overcome technical difficulties, Increased stability and easy filtration and sterilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Table 2
[0072] Element Dosage (g) Concentration (wt%) weight ratio to aprepitant Aprepitant 2.34g 0.721 1 egg yolk lecithin 32.5g 10 13.9:1 ethanol 9g 2.773 3.8:1 Soybean oil 30.6g 9.429 13.1:1 sodium oleate 1.8g 0.555 0.77:1 sucrose 18g 5.546 7.7:1 Water for Injection 230.3g 70.976 98.4:1 Absolute ethanol* 30g / /
[0073] *Solvent added in formulation but removed during preparation of oil phase;
[0074] Preparation Process:
[0075] 1) Mix 2.34g of aprepitant and 32.5g of egg yolk lecithin, add 30g of ethanol, fill with nitrogen protection, and dissolve the mixture by heating and stirring for 30min at 65°C and 200rpm; place the mixture on a rotary evaporator , 65 ° C under reduced pressure rotary steaming for 10 min to remove ethanol; add 9 g of ethanol and 30.6 g of soybean oil for injection to the resulting solution, fill with nitrogen protection, continue heating and stirring at ...
Embodiment 2
[0082] table 3
[0083] Element Dosage (g) Concentration (wt%) weight ratio to aprepitant Aprepitant 2.34g 0.721 1 egg yolk lecithin 29.2g 9 12.5:1 ethanol 9g 2.773 3.8:1 Soybean oil 30.6g 9.429 13.1:1 sodium oleate 1.8g 0.555 0.77:1 sucrose 18g 5.546 7.7:1 Water for Injection 233.5g 71.976 99.8:1 Absolute ethanol* 30g / /
[0084] *Solvent added in formulation but removed during preparation of oil phase;
[0085] Preparation Process:
[0086] 1) Mix 2.34g of aprepitant and 29.2g of egg yolk lecithin, add 30g of ethanol, fill with nitrogen protection, and dissolve the mixture by heating and stirring for 30min at 65°C and 200rpm; place the mixture on a rotary evaporator , 65 ° C under reduced pressure rotary steaming for 10 min to remove ethanol; add 9 g of ethanol and 30.6 g of soybean oil for injection to the resulting solution, fill with nitrogen protection, continue heating and stirring at 6...
Embodiment 3
[0102] table 5
[0103] Element Dosage (g) Concentration (wt%) weight ratio to aprepitant Aprepitant 2.34g 0.721 1 egg yolk lecithin 32.5g 10 13.8:1 Soybean oil 30.6g 9.429 13.1:1 sodium oleate 1.8g 0.555 0.77:1 sucrose 18g 5.546 7.7:1 Water for Injection 239.3g 73.749 102.3:1 Absolute ethanol* 30g / /
[0104] *Solvent added in formulation but removed during preparation of oil phase;
[0105] Preparation Process:
[0106] 1) Mix 2.34g of aprepitant and 32.5g of egg yolk lecithin, add 30g of ethanol, fill with nitrogen protection, and dissolve the mixture by heating and stirring for 30min at 65°C and 200rpm; place the mixture on a rotary evaporator , 65 ° C under reduced pressure rotary steaming for 30 min, remove ethanol; add 30.6 g of soybean oil for injection to the resulting solution, fill with nitrogen protection, continue heating and stirring at 65 ° C and 200 rpm for 10 min to prepare the oil ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric potential | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com