Gold complex containing diphosphine ortho-position carborane ligand as well as preparation method and application of gold complex
A gold complex and carborane technology, which is applied in the field of complex synthesis, can solve the problems of high catalyst equivalent, catalyst instability, and long reaction time, and achieve low catalyst consumption, simple and green preparation method, and fast reaction rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
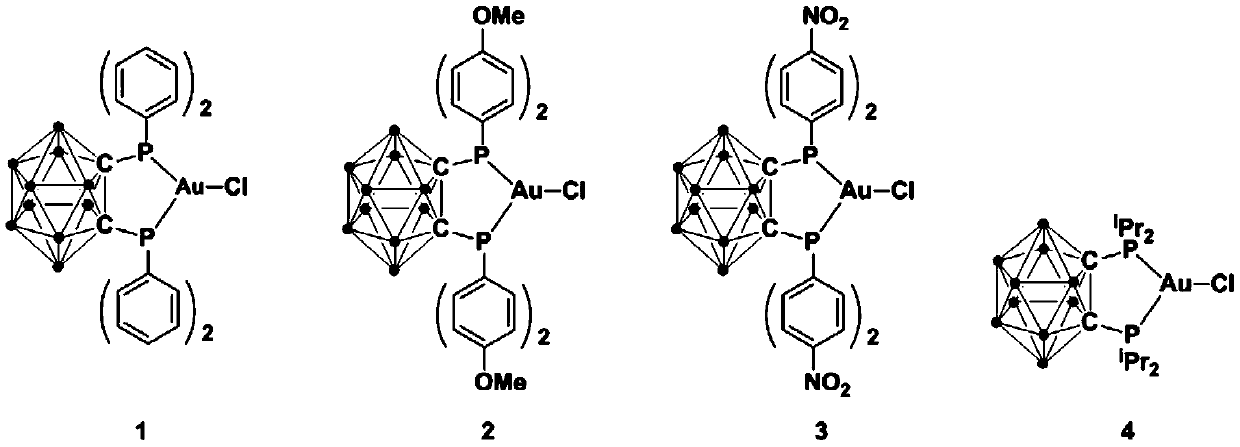
[0032] Example 1: Synthesis of gold complex 1 and its application in the synthesis of 2,4-disubstituted thiazole compounds by catalyzing the reaction of terminal alkyne and thioamide
[0033] At 0℃, add n-BuLi (2.2mmol) n-hexane solution dropwise to o-C containing ortho carborane 2 B 10 H 12 (1.0mmol) in ether solution, continue to stir for 30 minutes after the dropwise addition, slowly rise to room temperature and continue to react for 30 minutes, then add halophosphine ClPPh 2 (2.2mmol), continue to react at room temperature for 2 hours, and then add AuCl (1.0mmol) to the reaction system at room temperature and continue to react for 3 hours. After the reaction is over, stand still and filter, drain the solvent under reduced pressure, and wash the crude product with ether , And drain to obtain the target product 1 (yield 85%), the reaction formula is:
[0034]
[0035] 1 H NMR(400MHz, CDCl 3 , 25°C): δ=7.79-7.67 (m, 8H), 7.55-7.47 (m, 12H). Elemental analysis theoretical value C 2...
Embodiment 2
[0041] Example 2: Synthesis of gold complex 2
[0042] At 0℃, add n-BuLi (2.5mmol) n-hexane solution dropwise to o-C containing ortho carborane 2 B 10 H 12 (1.0mmol) in the ether solution, after the dropwise addition, continue to stir for 30 minutes, slowly rise to room temperature and continue the reaction for 30 minutes, then add the halogenated phosphine ClP(4-MeO-C 6 H 4 ) 2 (2.5mmol), continue to react at room temperature for 2 hours, and then add AuCl (1.0mmol) to the reaction system at room temperature and continue to react for 5 hours. After the reaction is over, stand still and filter, drain the solvent under reduced pressure, and wash the crude product with ether , And drain to obtain the target product 2 (yield 80%), the reaction formula is:
[0043]
[0044] 1 H NMR(400MHz, CDCl 3 , 25°C): δ=7.72-7.65 (m, 8H), 7.60-7.51 (m, 8H), 3.36 (s, 12H). Elemental analysis theoretical value C 30 B 10 H 38 O 4 P 2 AuCl: C 41.65, H 4.43; experimental value: C 41.58, H 4.51.
Embodiment 3
[0045] Example 3: Synthesis of gold complex 3
[0046] At 0℃, add n-BuLi (2.3mmol) n-hexane solution dropwise to o-C containing ortho carborane 2 B 10 H 12 (1.0mmol) in the ether solution, after the dropwise addition, continue to stir for 30 minutes, slowly rise to room temperature and continue the reaction for 30 minutes, and then add the halogenated phosphine ClP(4-NO 2 -C 6 H 4 ) 2 (3.0mmol), continue to react at room temperature for 2 hours, and then add AuCl (1.0mmol) to the reaction system at room temperature and continue to react for 4 hours. After the reaction is over, stand still and filter, drain the solvent under reduced pressure, and wash the crude product with ether , And drain to obtain the target product 3 (yield 81%), the reaction formula is:
[0047]
[0048] 1 H NMR(400MHz, CDCl 3 , 25°C): δ=7.82-7.75 (m, 8H), 7.64-7.57 (m, 8H). Elemental analysis theoretical value C 26 B 10 H 26 N 4 O 8 P 2 AuCl: C 33.76, H 2.83, N 6.06; experimental value: C 33.70, H 2.89, N 6.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com