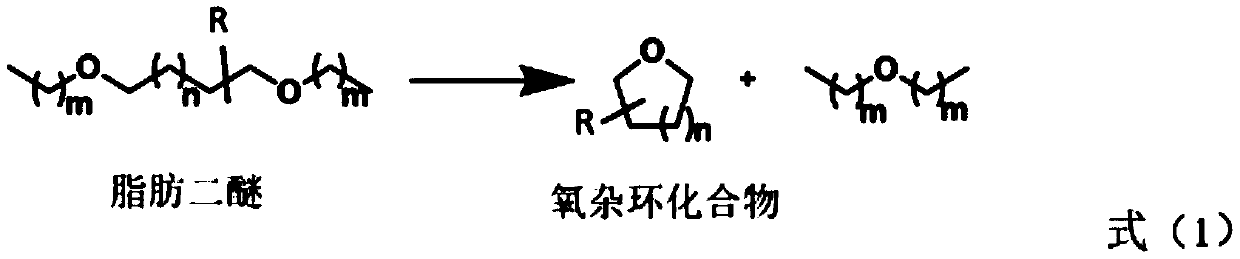
Novel method for preparing oxygen heterocyclic compound through ionic liquid catalysis
A technology of ionic liquids and compounds, applied in the field of catalysis, can solve problems such as unreported, and achieve the effects of no by-products, no metal participation, and simple separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
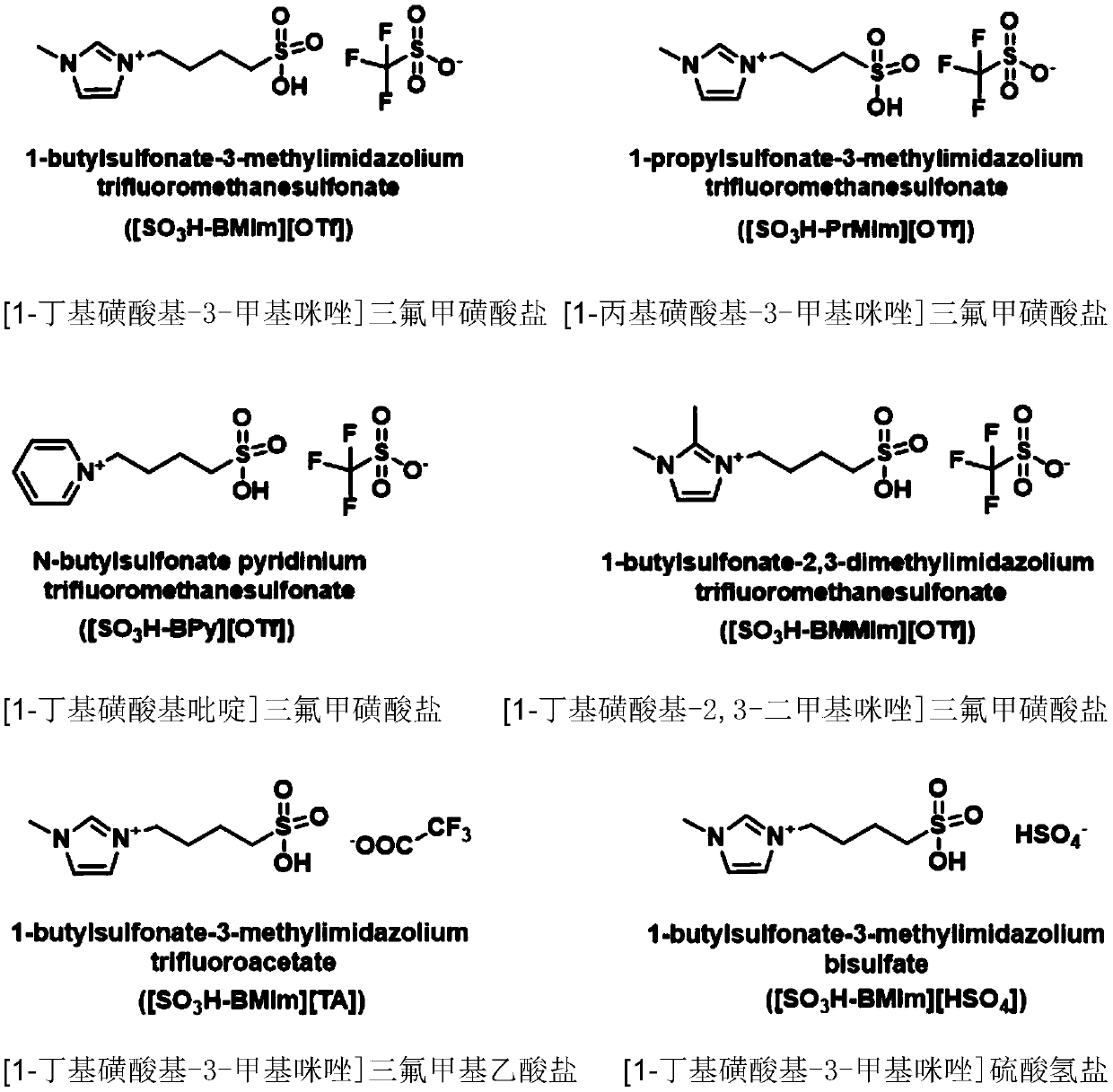
[0039] Example 1, [1-butylsulfonic acid group-3-methylimidazole] trifluoromethanesulfonate catalyzed 1,5-dimethoxypentane to prepare tetrahydropyran
[0040] Mix 2 mmol 1,5-dimethoxypentane with 0.2 mmol ionic liquid [1-butylsulfono-3-methylimidazole] trifluoromethanesulfonate ([SO 3 H-BMIm][OTf]) was placed in a 20 ml stainless steel reaction kettle, sealed; moved to an oil bath at 120°C, stirred and heated for 15 hours; the reaction kettle was immersed in ice water to terminate the reaction, and then left at room temperature for a period of time. The reaction solution was transferred to a separatory funnel and divided into upper and lower layers. Take the upper organic phase, use 1 H and 13 C NMR analysis of its composition and determination of material structure. According to the analysis results, it is determined that the conversion rate of raw materials is 94%, and the product is tetrahydropyran, and its separation yield is 94%.
[0041] reaction product 1 H and 13 ...
Embodiment 2
[0045] Example 2, [1-butylsulfonic acid group-3-methylimidazole] trifluoromethanesulfonate catalyzed 1,5-dimethoxypentane to prepare tetrahydropyran
[0046] Mix 0.1mol 1,5-dimethoxypentane with 0.1mol ionic liquid [SO 3 H-BMIm][OTf] was placed in a 50 ml stainless steel reactor and sealed; moved to an oil bath at 120°C, stirred and heated for 10 hours; the reactor was immersed in ice water to terminate the reaction, and then left at room temperature for a period of time. The reaction solution was transferred to a separatory funnel and divided into upper and lower layers. Take the upper organic phase, use 1 H and 13 C NMR analysis of its composition and determination of material structure. According to the analysis results, it is determined that the conversion rate of raw materials is >99.9%, the product is tetrahydropyran, and the separation yield is >99%.
Embodiment 3
[0047] Example 3, [1-propylsulfonate-3-methylimidazole] triflate catalyzes the preparation of tetrahydropyran from 1,5-dimethoxypentane
[0048] Mix 2 mmol 1,5-dimethoxypentane with 0.2 mmol ionic liquid [1-propylsulfono-3-methylimidazole] trifluoromethanesulfonate ([SO 3 H-PrMIm][OTf]) was placed in a 20 ml stainless steel reaction kettle, sealed; moved to an oil bath at 120°C, stirred and heated for 10 hours; the reaction kettle was immersed in ice water to terminate the reaction, and then left at room temperature for a period of time. The reaction solution was transferred to a separatory funnel and divided into upper and lower layers. Take the upper organic phase, use 1 H and 13 C NMR analysis of its composition and determination of material structure. According to the analysis results, it is determined that the conversion rate of raw materials is 90%, and the product is tetrahydropyran, and its separation yield is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com