Application of a kind of iridoid glycoside compound in preparation of β-glucuronidase inhibitor
An aldolidase and compound technology, applied in the field of biomedicine, can solve the problems of drug-induced diarrhea, affecting the chemotherapy process, delayed diarrhea, etc., and achieve the effect of significant inhibitory activity and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
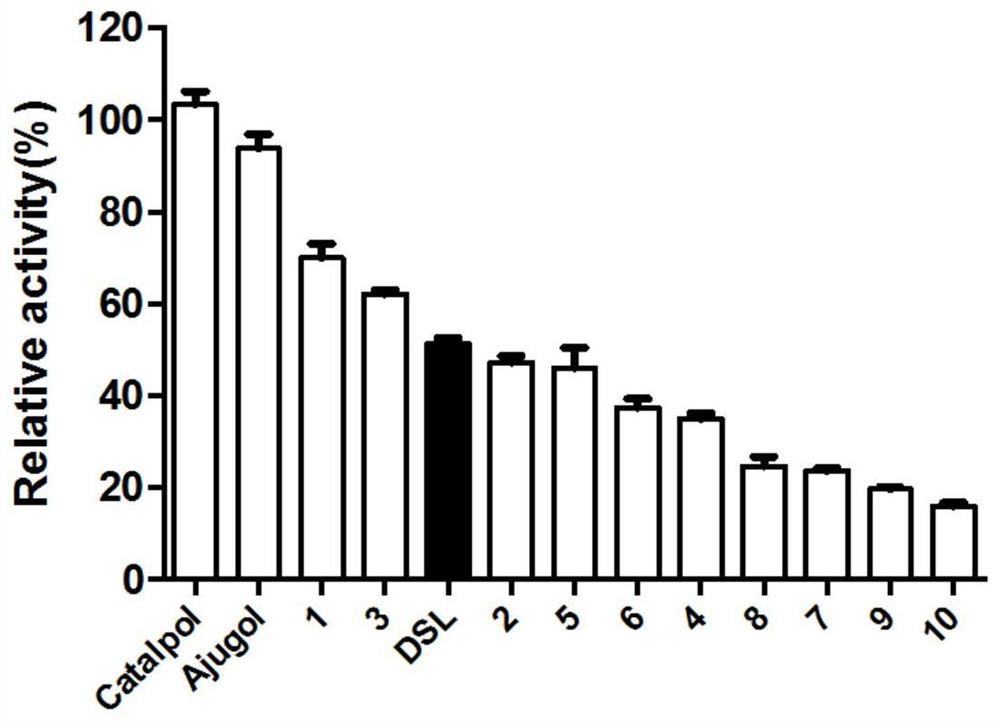
[0021] Example 1: Screening of EcGUS inhibitors
[0022] (1) Preparation of EcGUS:
[0023] Inoculate Escherichia coli BL21 (DE3) stored at -80°C into 200 mL of LB liquid medium containing 30 μg / mL kanamycin (trypsin 10 g / L, yeast extract 5 g / L, sodium chloride 10g / L, the solvent is water, pH7.0), cultivated at 200rpm, 37°C until the OD600 reaches 0.5, and then add isopropyl-β-D-thiogalactopyranoside (IPTG) at a final concentration of 100mM, Cultivate overnight at 200 rpm and 30°C to induce the expression of EcGUS (SDS-PAGE electrophoresis can detect the expression of the enzyme). After the expression is completed, centrifuge the culture solution at 4°C and 9000rpm for 5 minutes to collect the bacteria, then wash the bacteria with PBS (pH 7.4) 2-3 times, and then press the volume of the original bacteria solution (culture solution before centrifugation) 1 / 10 of lysate (20mM 4-hydroxyethylpiperazineethanesulfonic acid (HEPES), 300mM NaCl, 5mM imidazole (imidazole), volume con...
Embodiment 2
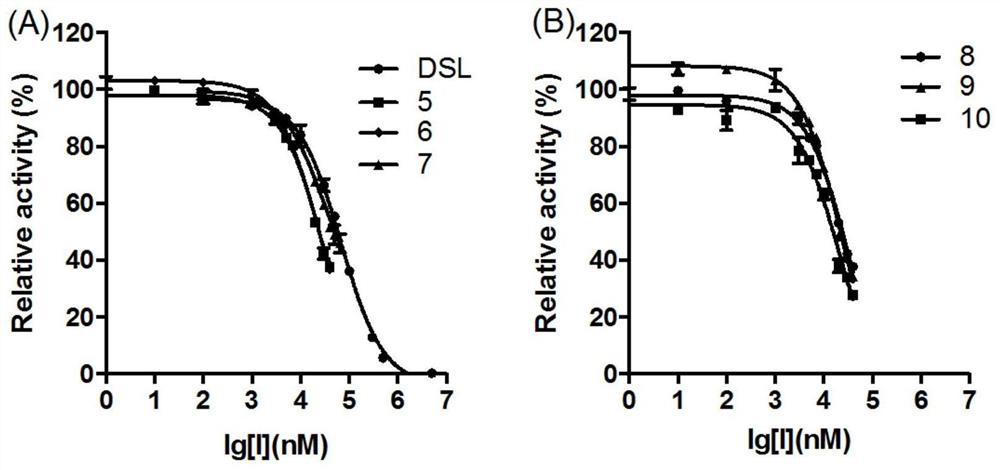
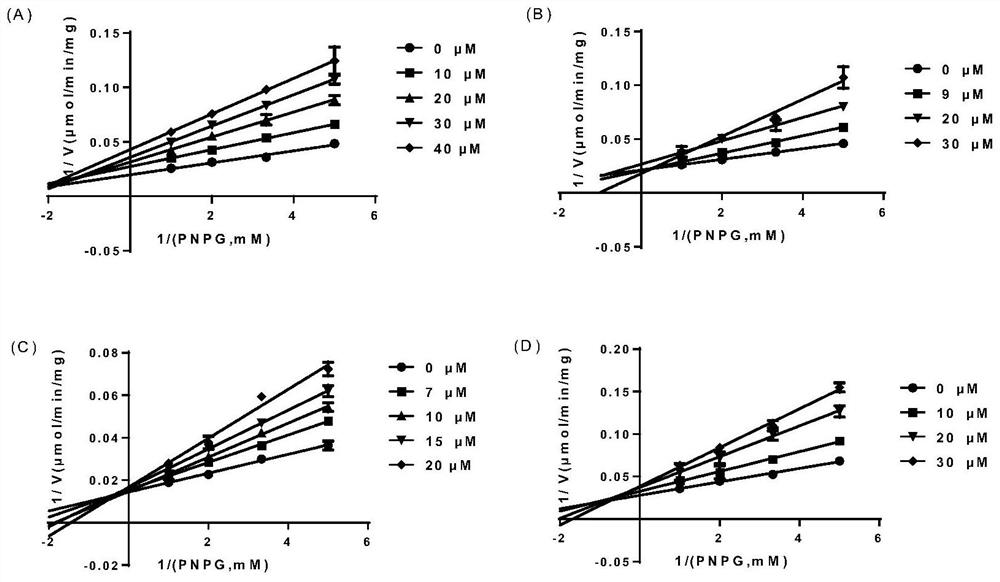
[0044] Example 2: Research on the type of inhibition of EcGUS by compounds 7, 8, 9, and 10
[0045] The four compounds with the best inhibitory activity in rescreening were selected, namely compounds 7, 8, 9, and 10. Their structures were all similar, and compound 9 had the best inhibitory activity. Inhibitors were prepared with PBS into solutions with a series of concentration gradients, and substrates were also prepared with PBS into solutions with concentrations of 2, 3, 5, and 10 mM, that is, the final concentrations were 200, 300, 500, and 1000 μM. Taking compound 7 as an example to make Table 2, the prepared solubility gradient is 0, 100, 200, 300, 400 μM, that is, the final concentration is 0, 10, 20, 30, 40 μM (see Table 2);
[0046] Table 2 The permutation and combination table of different solubility points of substrate and compound 7
[0047]
[0048] Note: C PNPG Indicates the final solubility of the substrate, C In Indicates the final solubility of the inhib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com