Method for preparing key intermediate of Remdesivir by using micro-channel reaction device
A channel reaction device and a technology for microchannel reaction, which are applied in the preparation of sugar derivatives, chemical instruments and methods, chemical/physical/physical-chemical reactors, etc., and can solve the problems of difficult industrial amplification, harsh reaction conditions, and low reaction efficiency. , to achieve the effect of good material mixing effect, simple operation and avoidance of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
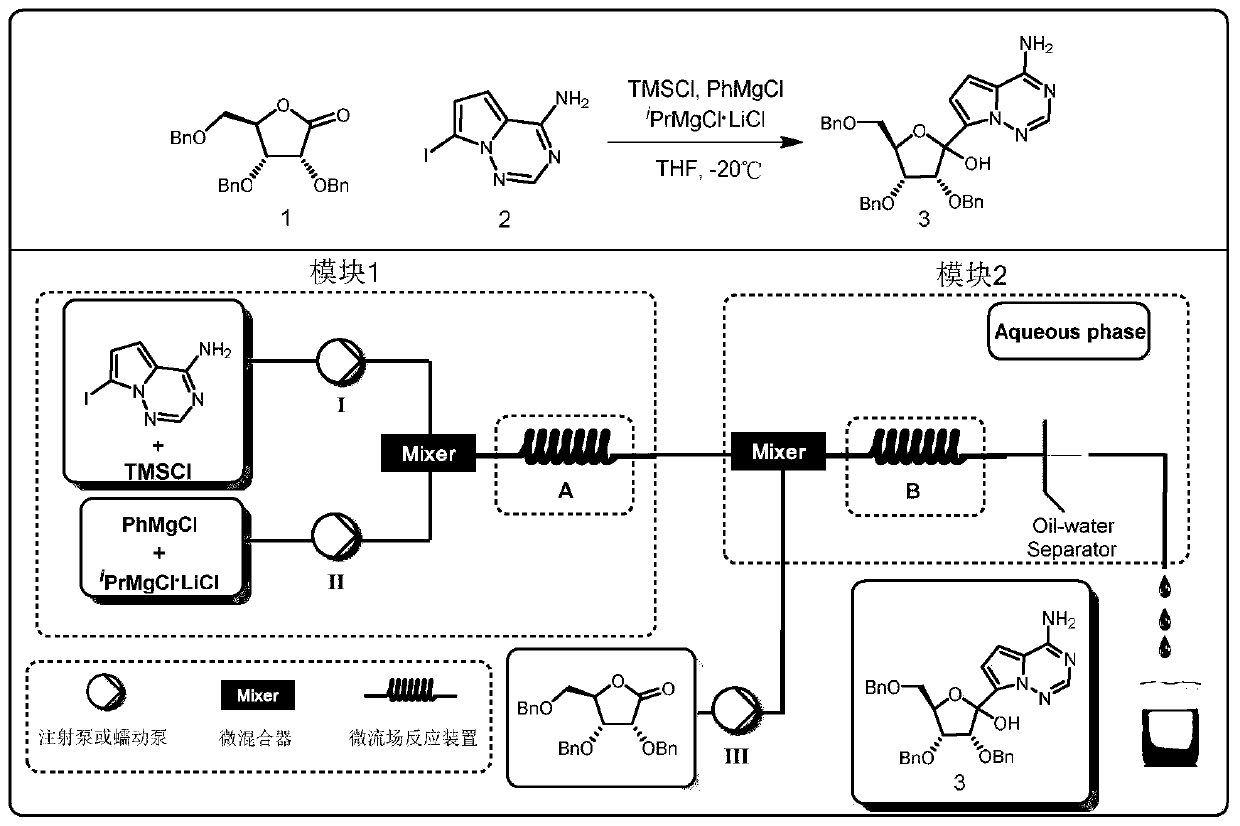
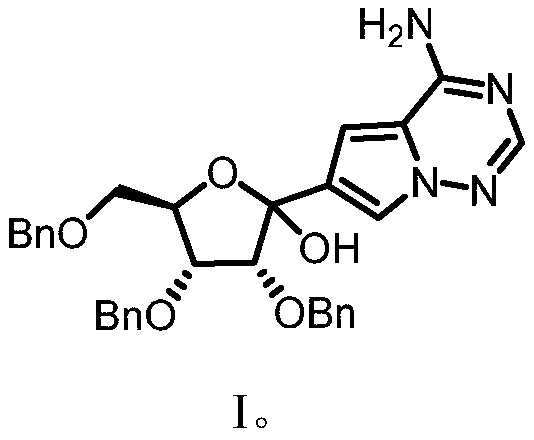
[0038] This example provides a method for preparing the key intermediate of remdesivir (3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4] using a microchannel reaction device Triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol, such as figure 1Shown, concrete synthesis method comprises the following steps:
[0039] Weigh 5.20g (20mmol, 1.0equiv) of 7-iodopyrrolo[2,1-f][1,2,4]triazin-4-amine, fully dissolve in tetrahydrofuran solvent, and add 5.0mL TMS-Cl (40mmol, 2.0equiv), prepared into 100mL solution, as material Ⅰ; measure PhMgCl 2 (2M, 40mmol, 2.0equiv) tetrahydrofuran solution 20mL and 20mL i PrMgCl 2 LiCl (1M, 20mmol, 1.0equiv) was mixed with THF solution as material II; 8.36g (20mmol, 1.0 equiv), fully dissolved in tetrahydrofuran solvent, prepared into 50mL solution, as material III. Simultaneously pump material I and material II, wherein, material I is pumped at a flow rate of 2.5 mL / min, and material II is pumped at a flow rate of 1.0 mL / min. Aft...
Embodiment 2
[0041] This example provides a method for preparing the key intermediate of Remdesivir (3R, 4R, 5R)-2-(4-aminopyrrolo[2,1-f][1,2,4] using a microchannel reaction device Triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol, such as figure 1 Shown, concrete synthesis method comprises the following steps:
[0042] Weigh 5.20g (20mmol, 1.0equiv) of 7-iodopyrrolo[2,1-f][1,2,4]triazin-4-amine, fully dissolve it in tetrahydrofuran solvent, and add 5.0mL TMS-Cl (40mmol, 2.0equiv), prepared into 80mL solution, as material Ⅰ; measure PhMgCl 2 (2M, 40mmol, 2.0equiv) tetrahydrofuran solution 20mL and 20mL i PrMgCl 2 LiCl (1M, 20mmol, 1.0equiv) was mixed with tetrahydrofuran solution as material II; 8.36g (20mmol, 1.0 equiv), fully dissolved in tetrahydrofuran solvent, prepared into 50mL solution, as material III. Simultaneously pump material Ⅰ and material Ⅱ, wherein, the pumping flow rate of material Ⅰ is 2.5 mL / min, and the pumping flow rate of material Ⅱ is 1...
Embodiment 3
[0044] This example provides a method for preparing the key intermediate of remdesivir (3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4] using a microchannel reaction device Triazin-7-yl)-3,4-bis(benzyloxy)-5-((benzyloxy)methyl)tetrahydrofuran-2-ol, such as figure 1 Shown, concrete synthesis method comprises the following steps:
[0045] Weigh 5.20g (20mmol, 1.0equiv) of 7-iodopyrrolo[2,1-f][1,2,4]triazin-4-amine, fully dissolve it in tetrahydrofuran solvent, and add 5.0mL TMS-Cl (40mmol, 2.0equiv), prepared into 50mL solution, as material Ⅰ; measure PhMgCl 2 (2M, 40mmol, 2.0equiv) tetrahydrofuran solution 20mL and 20mL i PrMgCl 2 LiCl (1M, 20mmol, 1.0equiv) was mixed with tetrahydrofuran solution as material II; 8.36g (20mmol, 1.0 equiv), fully dissolved in tetrahydrofuran solvent, prepared into 50mL solution, as material III. Simultaneously pump material I and material II, wherein, material I is pumped at a flow rate of 2.5 mL / min, and material II is pumped at a flow rate of 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com