Pharmaceutical composition for treating chronic atrophic gastritis and preparation method thereof
A technology of atrophic gastritis and composition, applied in the field of pharmaceutical preparations, can solve the problems of vicious circle, large side effects, strong dependence, etc., and achieve the effect of reversing gastric mucosal gland atrophy, safe medication and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A pharmaceutical composition for the treatment of chronic atrophic gastritis, which is prepared from the following raw materials in parts by weight: Codonopsis 18g, Poria 12g, Atractylodes 12g, Fig 13g, Tangerine peel 10g, Vinegar xiangfu 9g, Curcuma vinegar 9g, Perilla 8g stems, 11g ginger magnolia roots, 12g salvia miltiorrhiza, 13g soil turtles, 13g fried chicken's inner gold, 9g fried hawthorn, 3g roasted licorice.
[0043] The above-mentioned pharmaceutical composition is granulated according to a conventional method, filled, and prepared into capsules to obtain a capsule preparation that meets the daily dosage of a normal adult.
[0044] The above-mentioned capsule preparation met the requirements through the animal toxicity test, and did not cause damage or death of mice.
[0045] The above-mentioned capsule preparation is used for the treatment of patients with clinical chronic atrophic gastritis, and the method is as follows:
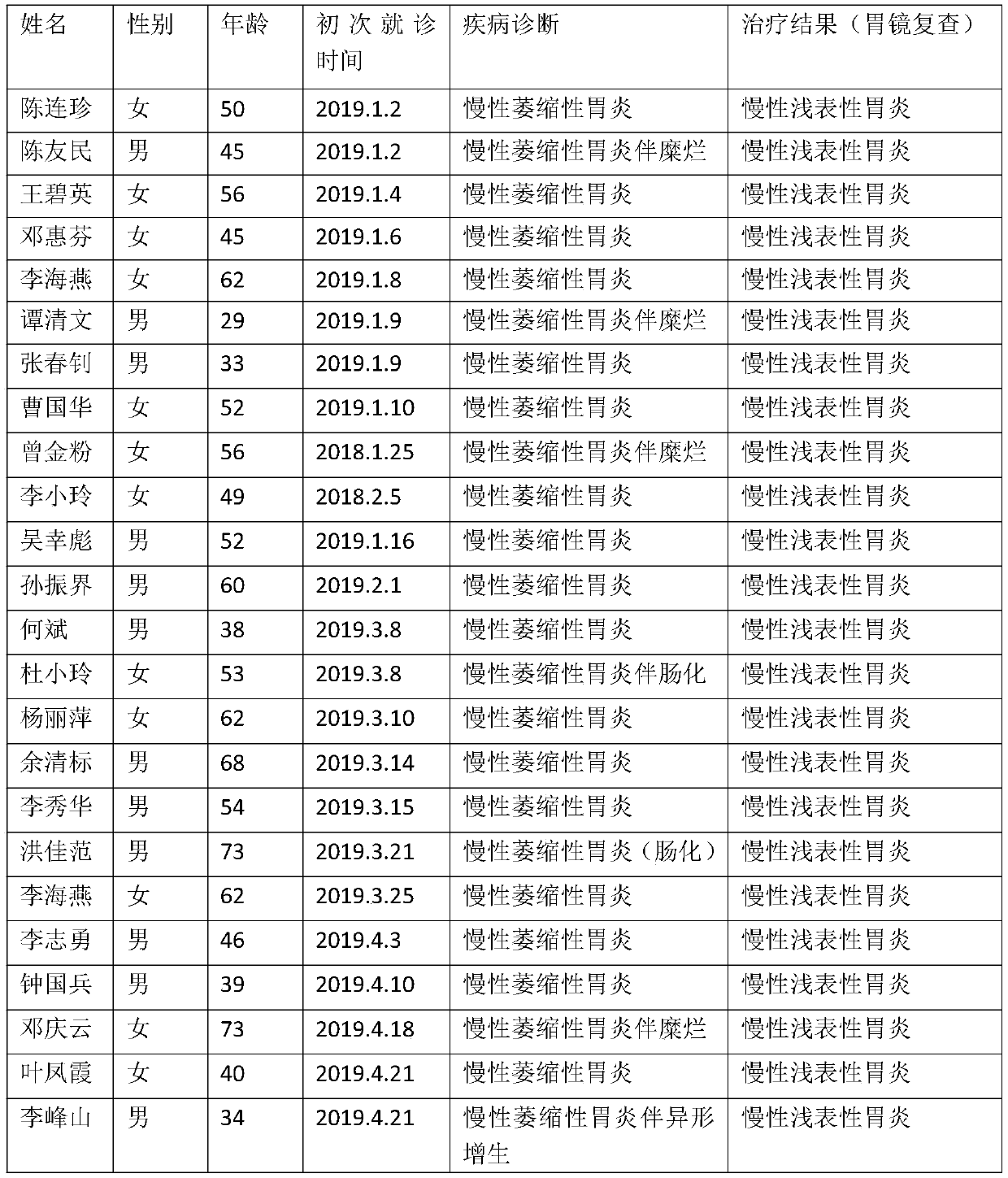
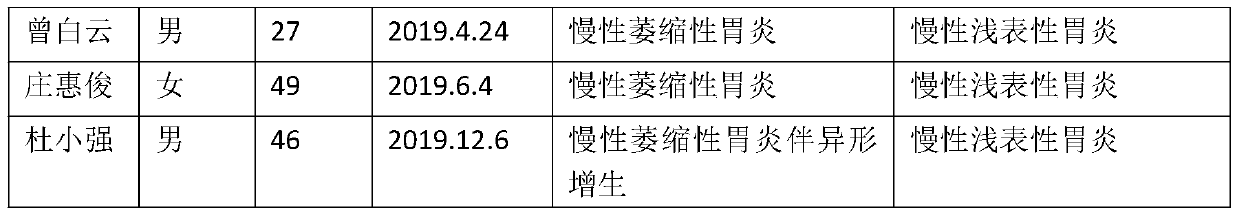
[0046] (1) Case data
[0047] There are 10...
Embodiment 2
[0060] A pharmaceutical composition for the treatment of chronic atrophic gastritis, prepared from the following raw materials in parts by weight: Codonopsis 24g, Poria 16g, Atractylodes 16g, Fig 18g, Tangerine peel 16g, Vinegar odorata 13g, Curcuma vinegar 13g, Perilla 12g stems, 17g ginger magnolia roots, 16g salvia miltiorrhiza, 16g soil turtles, 17g fried chicken's inner gold, 12g fried hawthorn, 6g roasted licorice.
[0061] The above-mentioned pharmaceutical composition is made into tablets and carried out in accordance with conventional methods to obtain tablets that meet the daily dosage of normal adults for the treatment of patients with clinical chronic atrophic gastritis. The method is as follows:
[0062] (1) Case data
[0063] There are 100 cases of chronic atrophic gastritis, including 50 males and 50 females, aged between 25-75 years old.
[0064] (2) Treatment method
[0065] The capsule preparation prepared above is taken orally, divided into 2 times a day for 3 months...
Embodiment 3
[0072] A pharmaceutical composition for the treatment of chronic atrophic gastritis, which is prepared from the following raw materials in parts by weight: Codonopsis 20g, Poria 15g, Atractylodes 15g, Fig 15g, Tangerine peel 15g, Vinegar fragrant root 10g, Curcuma vinegar 10g, Perilla 10g stems, 15g ginger magnolia roots, 15g salvia miltiorrhiza, 15g soil turtles 15g, fried chicken's inner gold 15g, fried hawthorn 10g, roasted licorice 5g.
[0073] The above-mentioned pharmaceutical composition is made into tablets and prepared according to conventional methods to obtain tablets that meet the daily dosage of normal adults for the treatment of patients with clinical chronic atrophic gastritis. The method is as follows:
[0074] (1) Case data
[0075] There are 100 cases of chronic atrophic gastritis, including 50 males and 50 females, aged between 25-75 years old.
[0076] (2) Treatment method
[0077] The tablets prepared above were taken orally, divided into 2 times a day for 3 months...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com