Double-walled carbon nanoring material and preparation method and application thereof
A technology of carbon nanorings and p-phenylene, applied in the direction of carbon nanotubes, nanocarbons, luminescent materials, etc., can solve the problems of re-hybridization, topological defects, and no literature reports on the successful preparation of double carbon nanorings, etc., and achieve structural stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
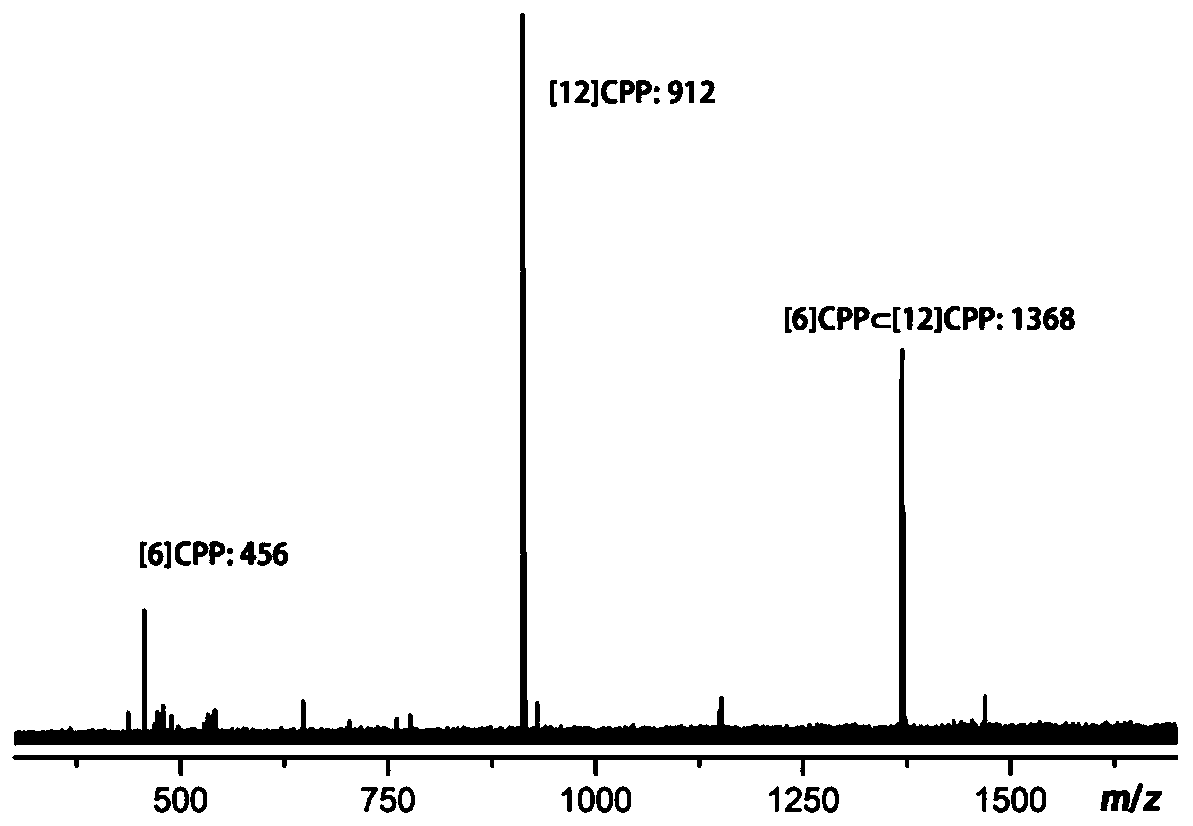
[0037] Mix the [12]CPP and [6]CPP-chlorobenzene solutions in the same volume according to the molar ratio of 1:1 (concentration: 5.0×10 -4 M), stirred and mixed at room temperature for 48 hours (150 rev / min), and light yellow double-walled carbon nanorings can be precipitated quickly after standing Centrifuge (8000 rpm, 5 minutes) and wash with a chlorobenzene solvent to obtain a pure double-walled carbon nanoring material, i.e. (also known as Complex).
[0038] Firstly, the product was characterized by mass spectrometry, and the results were as follows: figure 1 shown. Depend on figure 1 It can be seen that a very stable The molecular weight is 1368, and the mass spectrometer used is matrix-assisted laser desorption ionization mass spectrometry. Under laser irradiation, the molecular ion peak of the complex is still very strong.
[0039] The product was characterized by quantum chemical calculations, the results are as follows Figure 4 Shown, double-walled carbon ...
Embodiment 2
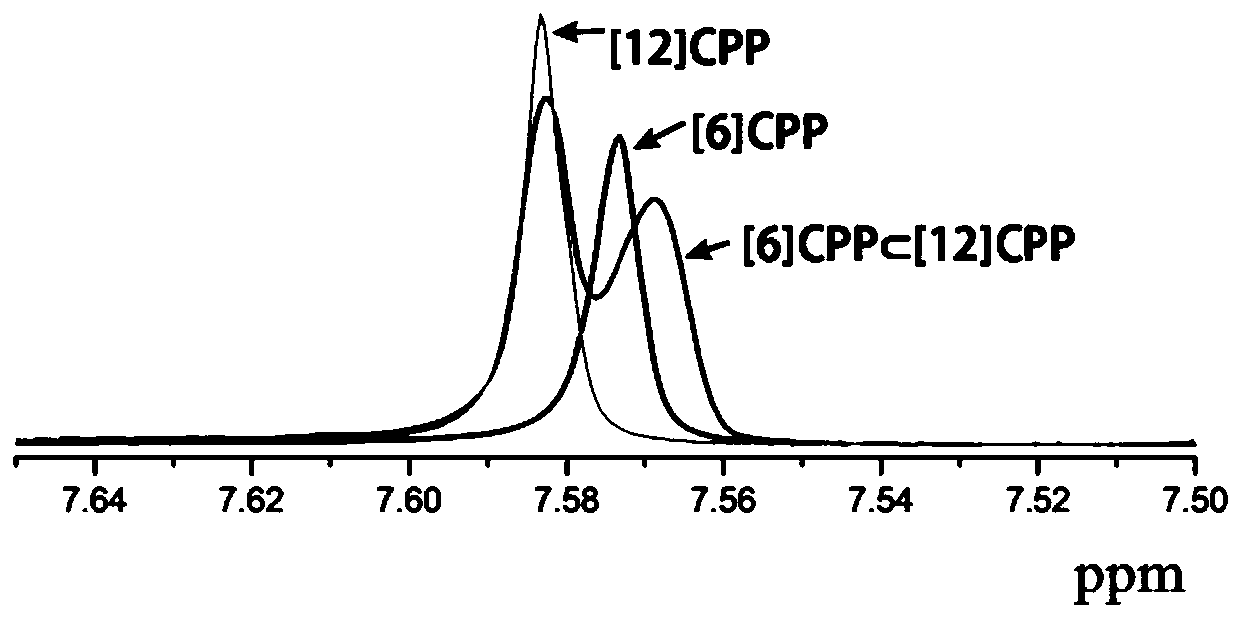
[0041] To the prepared in the embodiment of the present invention 1 Complex carried out 1 H NMR characterization.
[0042] The preparation concentration is 5.0×10 -4 M's [6] CPP monomer, [12] CPP monomer and prepared in Example 1 of the present invention The deuterated dichloromethane solution of the complex was 0.5 mL each, and the solution was transferred into three nuclear magnetic tubes, and then characterized by hydrogen nuclear magnetic spectrum. figure 2 Prepared for [6] CPP monomer, [12] CPP monomer and Example 1 of the present invention Composite 1 H NMR characterization chart. Depend on figure 2 It can be seen that the prepared in Example 1 of the present invention Composite 1 The overall intensity of the H NMR peak decreases, and in addition, the prepared in Example 1 of the present invention The fingerprint peak of [6]CPP in the complex has shifted to the right by about 0.005ppm, which shows that the compound prepared in Example 1 of the present inv...
Embodiment 3
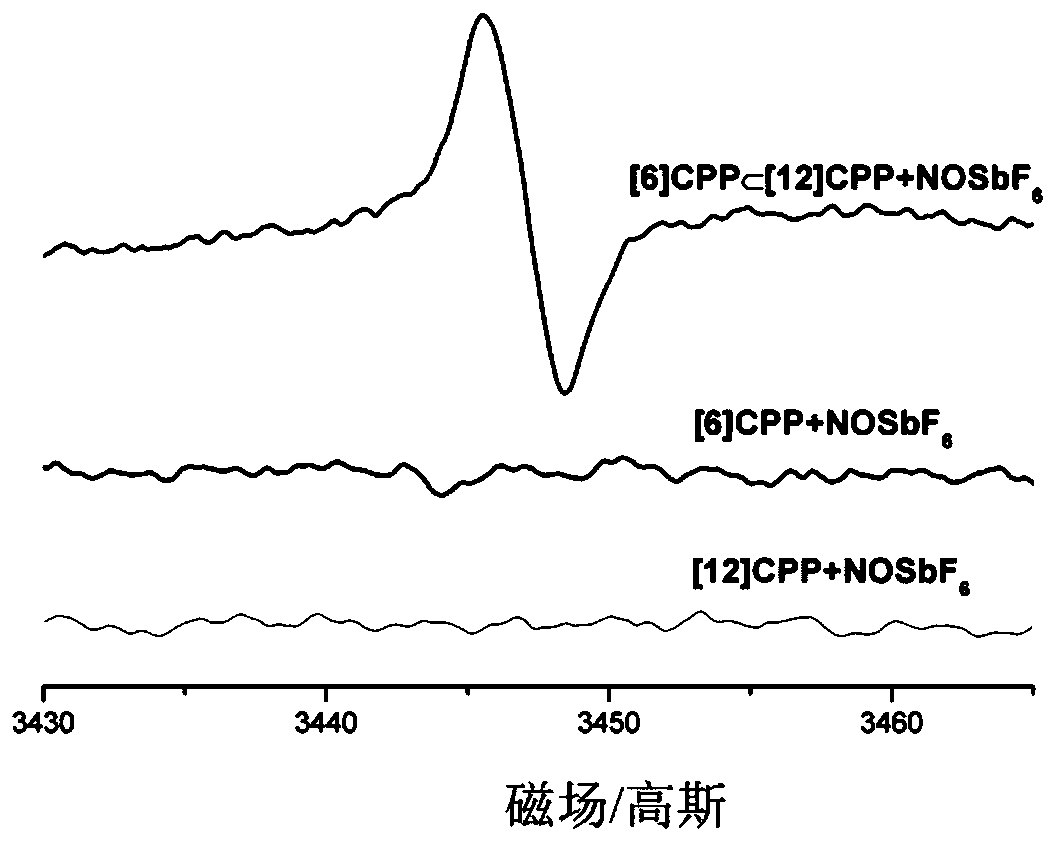
[0044] To the prepared in the embodiment of the present invention 1 The magnetic properties of cations were studied.
[0045] Each preparation [6] CPP monomer, [12] CPP monomer and the prepared in the embodiment of the present invention 1 The dichloromethane solution of the complex (5.0×10 -5 M). The oxidizing agent used is nitrous hexafluoroantimonate (NOSbF6), prepared 5.0×10 -5 M's oxidant solution. The oxidant solution and the carbon nanoring solution were transferred into a paramagnetic tube at a volume ratio of 1:1, ultrasonicated, and then characterized by electron paramagnetic resonance (EPR). pass image 3 It can be seen that [6] CPP monomer and [12] CPP monomer have no EPR signal, while the prepared in Example 1 of the present invention The complex presents a stronger free radical EPR signal, which indicates that the compound prepared in Example 1 of the present invention The cations of the complex are very stable, and the cations have paramagnetic prope...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com