Preparation method of bio-based polyester
A technology based on polyester and biology, applied in the field of preparation of bio-based polyester, to achieve the effect of controllable polymerization reaction and elimination of depolymerization reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
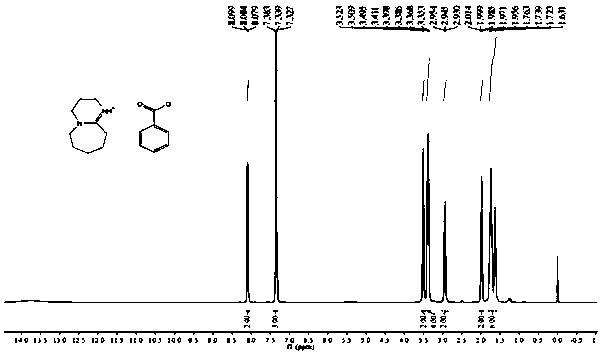
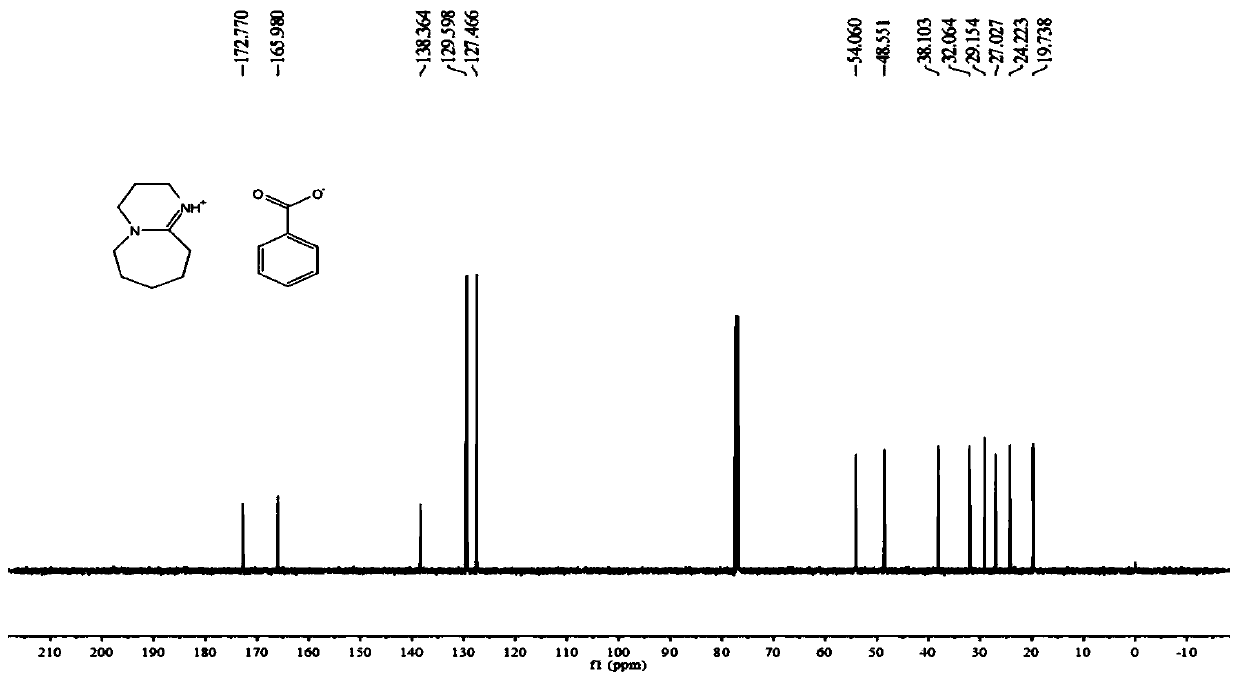
[0034] For the preparation of DBU·BA, dissolve benzoic acid in ether solution at room temperature under nitrogen or inert gas protection conditions, and drop DBU into the reaction flask while stirring to obtain the product. The hydrogen spectrum structure of DBU·BA is as follows figure 1 As shown, the carbon spectrum structure is as figure 2 shown.
[0035] The argon in the reaction kettle was replaced three times, and 19.4g of dimethyl terephthalate and 10.8ml of ethylene glycol (molar ratio of 1:2) were added under the state of argon, and the temperature was gradually raised to 250 ℃, carry out the condensation reaction until no methanol is distilled out. Add 0.028g DBU·BA salt (0.1% mol) and reduce the pressure to 0.3 atmosphere to carry out the transesterification reaction, raise the temperature to 280°C until no ethylene glycol evaporates. After the reaction was complete, cool to room temperature with nitrogen gas, and the product was a colorless transparent solid wit...
Embodiment 2
[0037] The DBU·BA catalyst was prepared as in Example 1.
[0038] Replace the argon in the reaction kettle three times, add 19.4g dimethyl terephthalate and 14.5ml 1,3-propanediol (molar ratio is 1:2) under the state of argon, and gradually increase the temperature under normal pressure To 250 ° C, the condensation reaction is carried out until no methanol is distilled out. Then add 0.028g of DBU·BA salt (0.1% mol), then reduce the pressure to 0.2 atmosphere to carry out the transesterification reaction, raise the temperature to 280°C until no ethylene glycol is distilled out. After the reaction was complete, the reaction was cooled to room temperature with nitrogen gas, and the product was a colorless transparent solid with a weight of 19.9 g and a yield of 60%.
Embodiment 3
[0040] The DBU·BA catalyst was prepared as in Example 1.
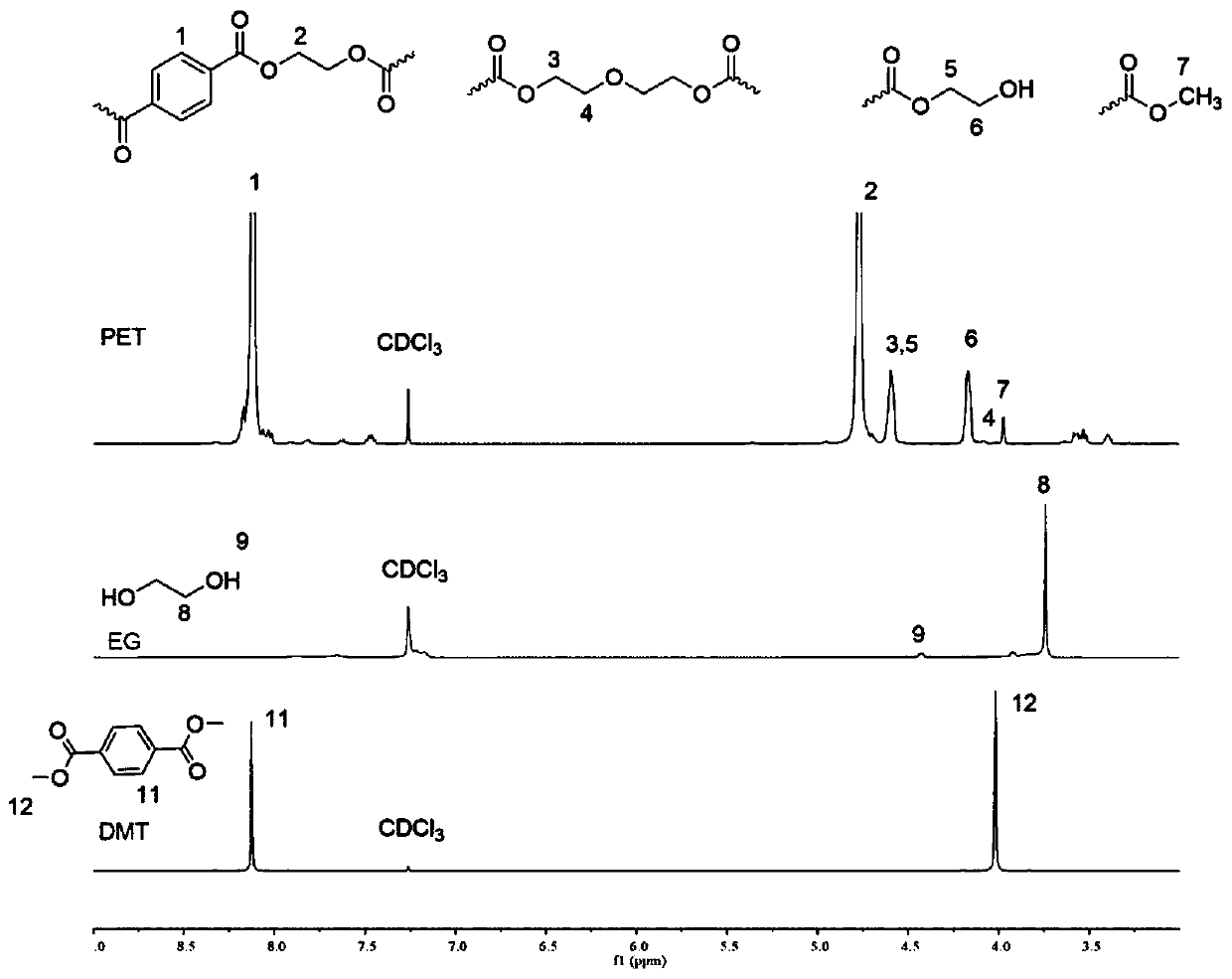
[0041] The argon in the reaction kettle was pumped three times, and 19.4g of dimethyl terephthalate and 17.7ml of 1,4-butanediol (molar ratio was 1:2) were added under the state of argon, and gradually increased under normal pressure. The temperature is up to 220°C, and the condensation reaction is carried out until no methanol is distilled out. Then add 1.12g of DBU·BA salt (4% mol), then reduce the pressure to 0.3 atmospheric pressure to carry out the transesterification reaction, raise the temperature to 280°C until no ethylene glycol evaporates. After the reaction is complete, the nitrogen gas is cooled to room temperature, and the product is a colorless, transparent to pale yellow solid, with a product weight of 25.3 g and a yield of 65%. The product PBT and its raw materials are as follows: Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com