A kind of copper-doped bismuth bimetallic material and its preparation and application
A bismuth metal and copper doping technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve poor stability, low current density, and low Faradaic efficiency and other issues, to achieve the effect of low cost, good conductivity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of CuBi electroplating solution under constant current density, described CuBi electroplating solution is used for electrodeposition Cu-doped-Bi metal electrode, and preparation method is:
[0043] Take 0.01mol trisodium citrate dihydrate, 0.01mol urea, 0.01mol ethylenediaminetetraacetic acid, 0.001mol Bi(NO 3 ) 3 ·5H 2 O and 0.001mol Cu(NO 3 ) 2 ·3H 2 O was successively dissolved in 100 mL deionized water. After stirring well, use 0.01M HNO 3 and 0.01M NaOH to adjust the pH to 6, add 0.68g sodium formate, stir well until clear and sonicate.
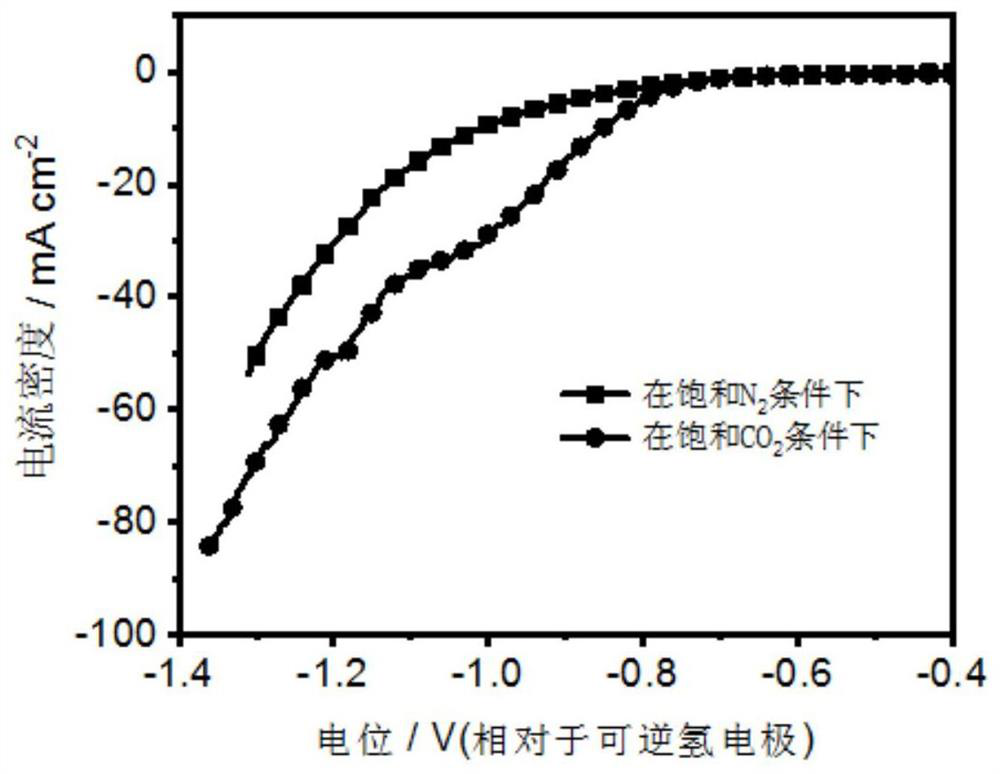
[0044] The pretreated foamed copper was cut into 1cm×1.5cm as the working electrode, and the spectroscopic graphite rod was used as the counter electrode, and the Cu-doped-Bi metal electrode for the electrochemical reduction of carbon dioxide was obtained by electrodeposition at 400mA for 1min. , called Cu (1) -doped-Bi (1) -400.
Embodiment 2
[0046] The preparation method of CuBi electroplating solution under constant current density, described CuBi electroplating solution is used for electrodeposition Cu-doped-Bi metal electrode, and its preparation method is:
[0047] Take 0.01mol trisodium citrate dihydrate, 0.01mol urea, 0.01mol ethylenediaminetetraacetic acid, 0.001mol Bi(NO 3 ) 3 ·5H 2 O and 0.00025mol Cu(NO 3 ) 2 ·3H 2 O was successively dissolved in 100 mL deionized water. After stirring well, use 0.01M HNO 3 and 0.01M NaOH to adjust the pH to 6, add 0.68g sodium formate, stir well until clear and sonicate.
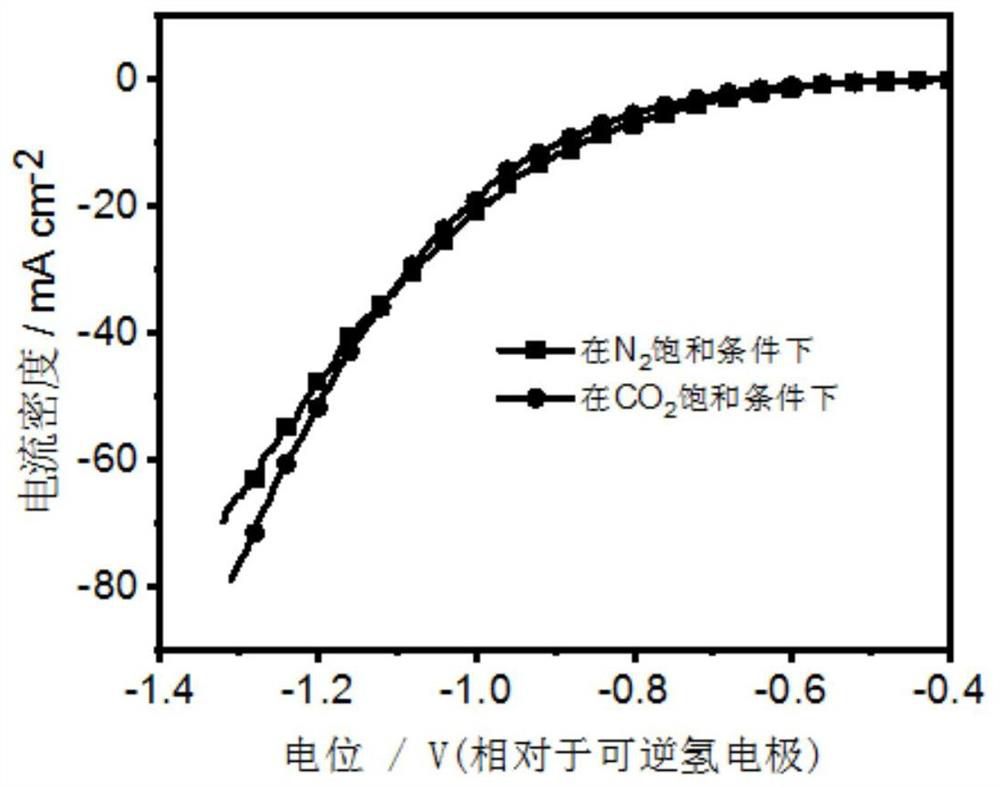
[0048] The pretreated foamed copper was cut into 1cm×1.5cm as the working electrode, and the spectroscopic graphite rod was used as the counter electrode, and the Cu-doped-Bi metal electrode for the electrochemical reduction of carbon dioxide was obtained by electrodeposition at 400mA for 1min. , called Cu (0.25)- doped-Bi (1) -400.
Embodiment 3
[0050] Preparation method of CuBi electroplating solution under constant current density. Described CuBi electroplating solution is used for electrodeposition Cu-doped-Bi metal electrode, and its preparation method is:
[0051] Take 0.01mol trisodium citrate dihydrate, 0.01mol urea, 0.01mol ethylenediaminetetraacetic acid, 0.001mol Bi(NO 3 ) 3 ·5H 2 O and 0.00025mol Cu(NO 3 ) 2 ·3H 2 O was successively dissolved in 100 mL deionized water. After stirring well, use 0.01M HNO 3 and 0.01M NaOH to adjust the pH to 6, add 0.68g sodium formate, stir well until clear and sonicate.
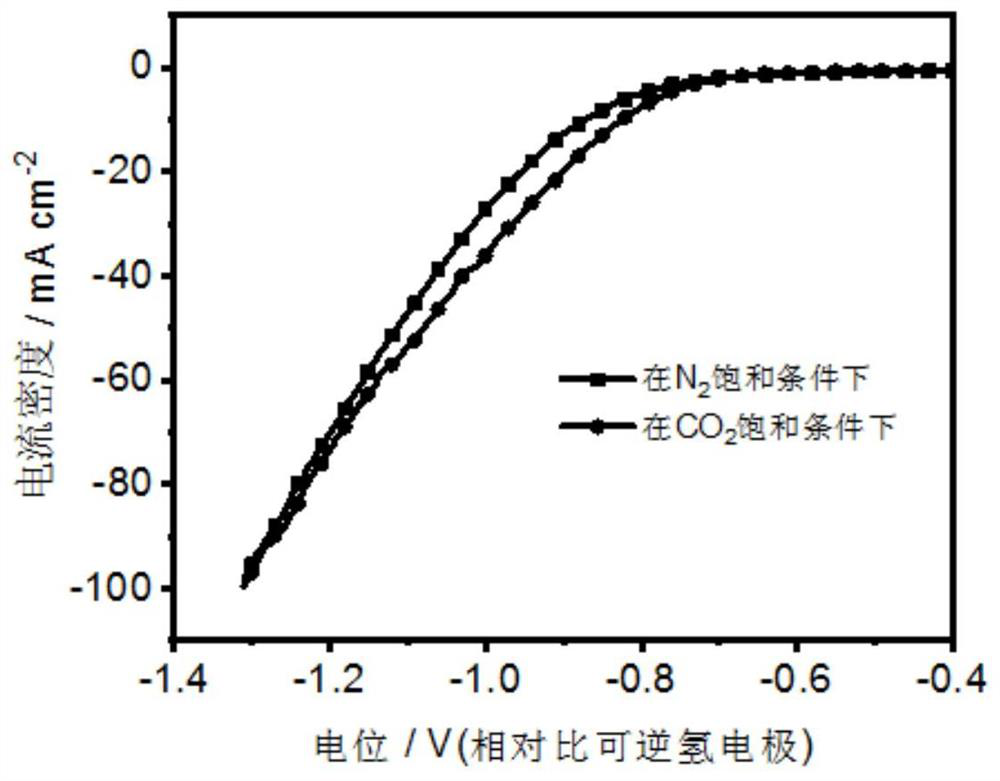
[0052] The pretreated foamed copper was cut into 1cm×1.5cm as the working electrode, and the spectroscopic graphite rod was used as the counter electrode, and the Cu-doped-Bi metal electrode for the electrochemical reduction of carbon dioxide was obtained under the condition of 100mA for 1min. , called Cu (0.25)- doped-Bi (1) -100.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com