Compositions and methods for restoring or preventing loss of vision caused by disease or traumatic injury
A technology for diseases and retinal damage, applied in drug combinations, sensory diseases, organic active ingredients, etc., can solve problems such as task complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
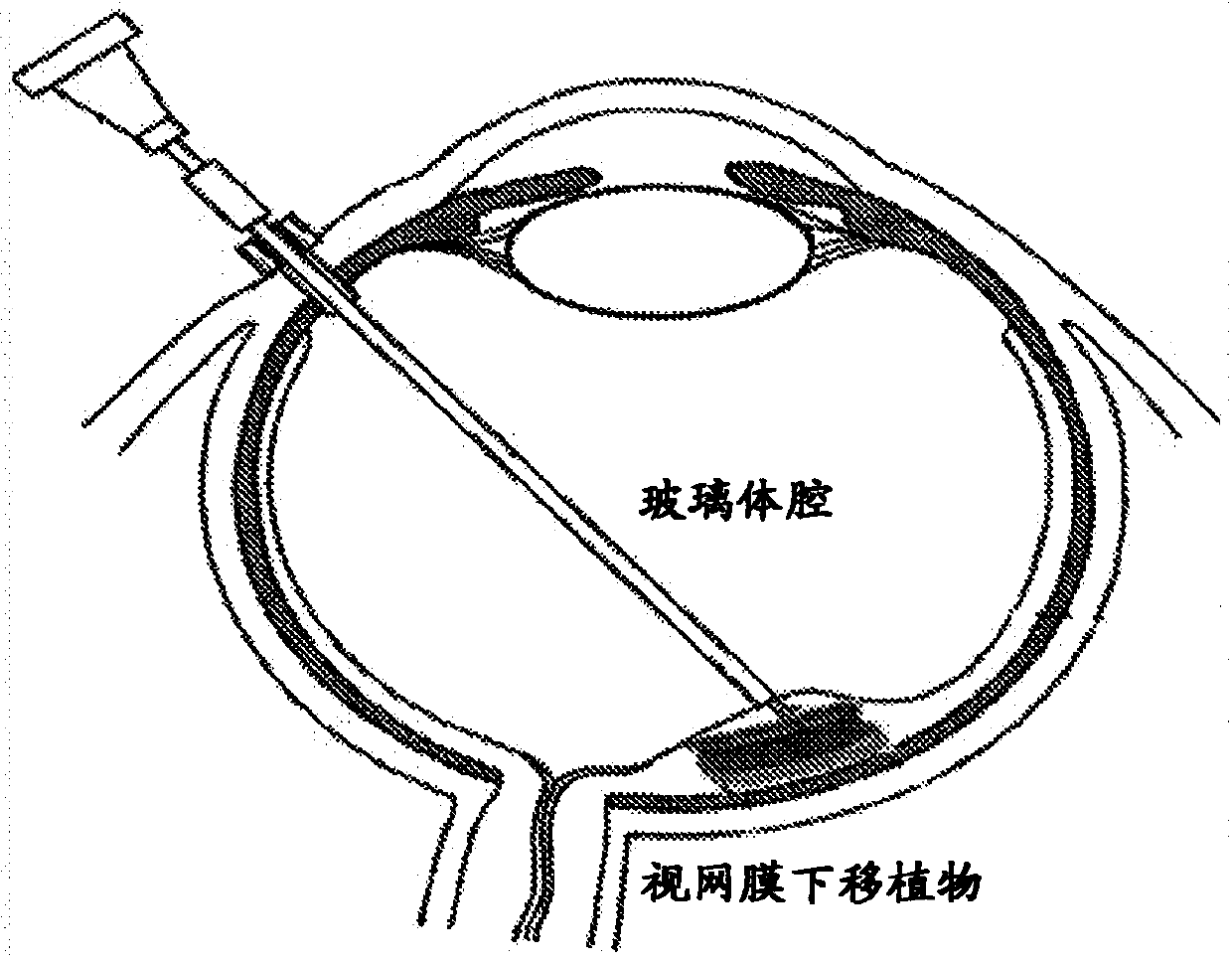
[0227] Restoration and improvement in visual perception was demonstrated in rabbits with ocular impact exposure and retinal damage. Subretinal grafts containing only hESC-3D retinal tissue (no biomaterials / scaffolds) were used to treat damaged retinal tissue in rabbits. Following sacrifice, optical coherence tomography (OCT) and histology and immunohistochemistry in live animals were used to demonstrate structural recovery of tissue and vision. Functional recovery was demonstrated in live animals using visual evoked potentials (VEP).
[0228] Human retinal tissue was generated using clinical grade hPSCs (BIOTIME, INC.). Pilot transplantation experiments were performed in rabbits to determine the subretinal transplantation process in a large eye animal model. An ocular impact injury model was generated in rabbits using a shock tube. Multiple pieces of hESC-3D retinal tissue (between about 0.1 and about 1 mm in length) were then transplanted into the subretinal space of each ...
Embodiment 2
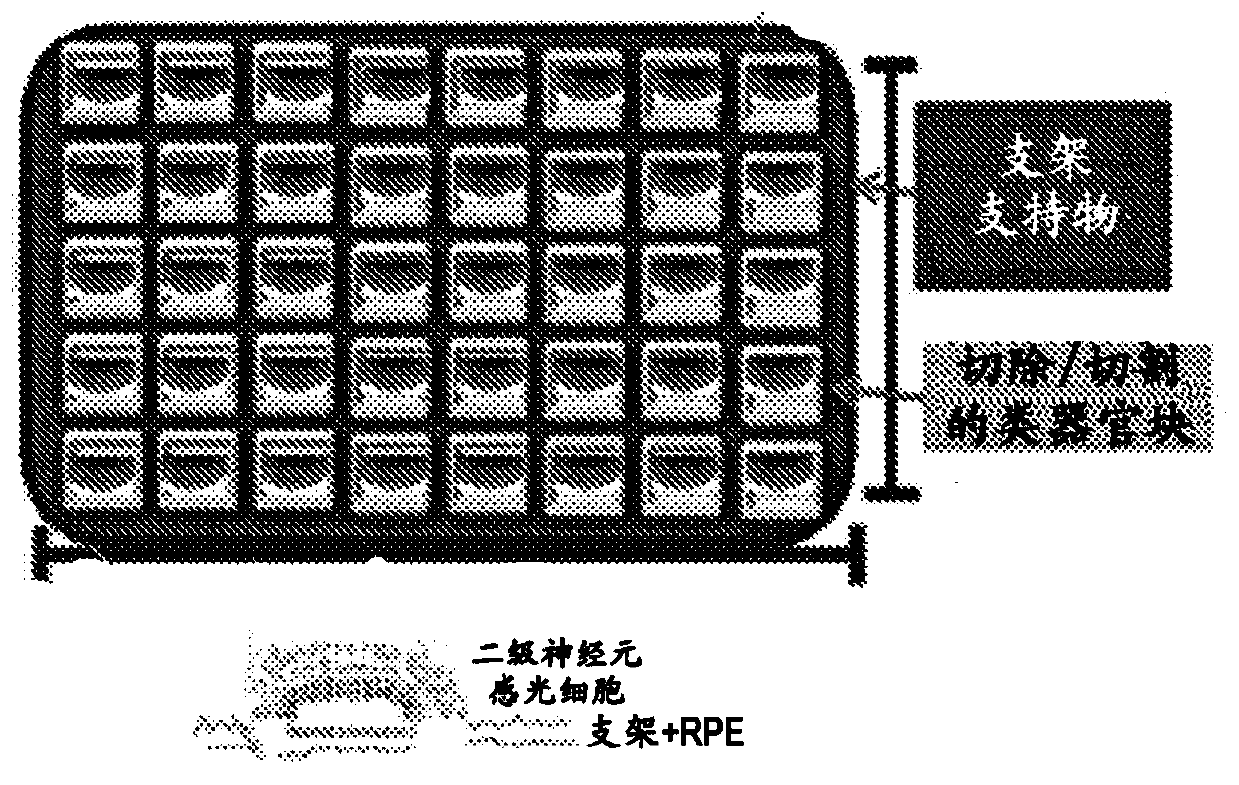
[0235] Visual recovery and improvement was demonstrated in rabbits with ocular impact exposure and retinal damage. Subretinal grafts comprising hESC-3D retinal tissue and biodegradable and / or non-biodegradable carriers or scaffolds were used to treat damaged retinal tissue in rabbits. As described herein, subretinal grafts may comprise hESC-3D retinal tissue blocks mounted on thin electrospun nanofiber layers of biomaterial scaffolds to form biological retinal patches. Following sacrifice, optical coherence tomography (OCT) and histology and immunohistochemistry in live animals were used to demonstrate structural recovery of tissue and vision. Functional recovery was demonstrated in live animals using visual evoked potentials (VEP).
[0236] Human retinal tissue was generated using clinical grade hPSCs (BIOTIME, INC.). Pilot transplantation experiments were performed in rabbits to determine the subretinal transplantation process in a large eye animal model. An ocular impact...
Embodiment 3
[0241] The hPSC-retinal progenitors were delivered into the ocular space of rabbits using an eye syringe (ex vivo experiments). Frozen sections of rabbit eyes engrafted with human retinal progenitor cells were stained with anti-human nuclear antibody HNu (red) and whole-nucleus DAPI staining (blue). The presence of human retinal cells (red + blue staining) in the rabbit eye space (blue staining only) delivered by means of an ocular syringe was demonstrated. Figure 5B to Figure 5D Demonstrated that subretinal grafts of human retinal progenitor cells differentiated from human embryonic stem cells (hESCs) can be successfully transplanted into the ocular space of an animal model of megaphthalmia (rabbit), maintaining retinal layer thickness in the adult mammalian retina Up to 3 months, no harmful effects on the recipient retina, and no tumorigenesis. Cells from these grafts migrated and integrated into the recipient retinal layers, strengthening the recipient retina. Such cells...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com