A chimeric antigen receptor modified T cell that can autocrine tlr4 scFv and targets cMet and its application
A technology of chimeric antigen receptors and chimeric receptors, which is applied in the field of T lymphocytes and its preparation, can solve problems such as the lack of research reports on T cell immunotherapy, so as to overcome the problem of tumor immune evasion, reduce the probability of escape, Effects that promote killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Synthesis of CAR-expressed genes
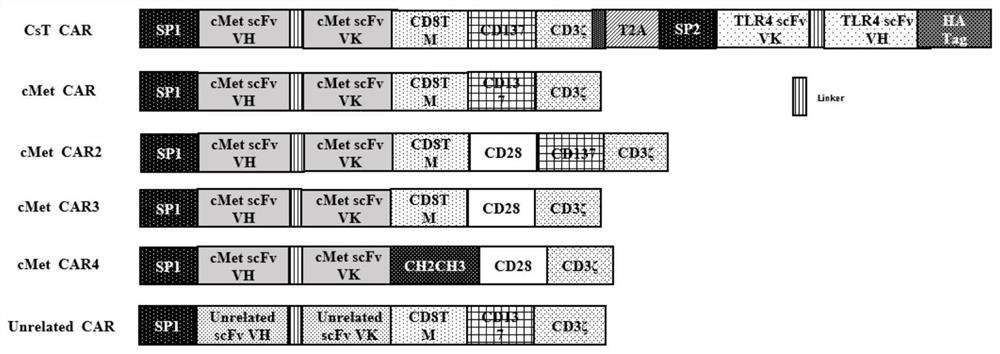
[0062] The bispecific chimeric antigen receptor provided by the present invention is composed of CD8α signal peptide, c-Met scFv, CD8 transmembrane region, CD137 intracellular signal region, CD3ζ intracellular signal region, T2A self-cleavage sequence, antibody secretion signal peptide VH3 and TLR4 scFv are formed in series, the structure is as follows figure 1 The gene sequence of anti-c-Met and TLR4 single-chain antibody (anti-c-Met scFv and TLR4 scFv) is derived from the monoclonal antibody sequence constructed in this study, and it is codon-optimized to ensure that the coding amino acid sequence remains unchanged. In this case, it is more suitable for the expression of T cells, and the sequence information of each gene is shown in SEQUENCE LISTING (SEQ ID NO. 1~18 of the sequence table).
Embodiment 2
[0063] Example 2: Construction of CAR plasmid
[0064] Use overlap PCR to complete the splicing of each structure of the CAR in Example 1, respectively, to obtain a CAR c-Met , secreted CAR1 c-Met / TLR4 , and the control CAR CD19 (Unrelated CAR).
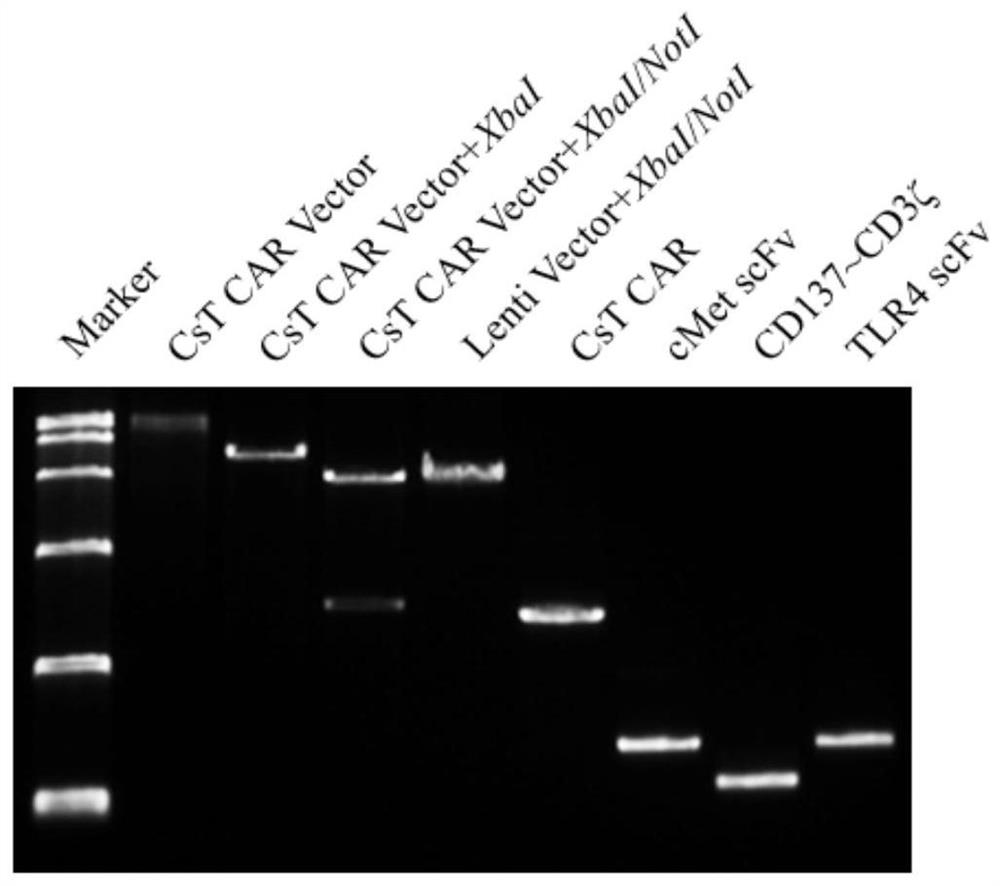
[0065] And add the Xba I restriction site at the 5' end, the Not I restriction site at the 3' end, and the EcoR I restriction site at the 5' end of T2A. The vector pCDH-CMV-MCS-EF1a-CopGFP was digested with Xba I / Not I double enzyme, and then the CAR was separated by In-fusion PCR method. c-Met Fragmented, secreted CAR c-Met t / TLR4 Fragment and control CAR CD19 The fragment was ligated with the pCDH-CMV-MCS-EF1a-CopGFP vector (see figure 2 ).
[0066] The product obtained from the ligation was transformed into E.coli (DH5α) competent, and after picking a single clone for cultivation, the plasmid was extracted and sequenced to obtain pCDH-CAR c-Met , pCDH-secreted CAR c-Met t / TLR4 and pCDH-CAR CD19 plasmid.
Embodiment 3
[0067] Example 3: Secretory-specific CAR binding ability and identification of exogenous CD3ζ expression
[0068] X-293T cells in logarithmic growth phase were collected 1D before transfection, and X-293T cells were seeded in 10cm cell culture dishes at a seeding volume of 10 7 , the cells were grown in DMEM medium containing 10% FBS, placed in a 37°C, 5% CO2 cell incubator and cultured in a cell incubator with a microscope to observe that the cell density reached 60-80% before transfection.
[0069] pCDH-CAR c-Met , pCDH-secreted CAR c-Met t / TLR4 and pCDH-CAR CD19 Plasmids were transfected into X-293T cells.
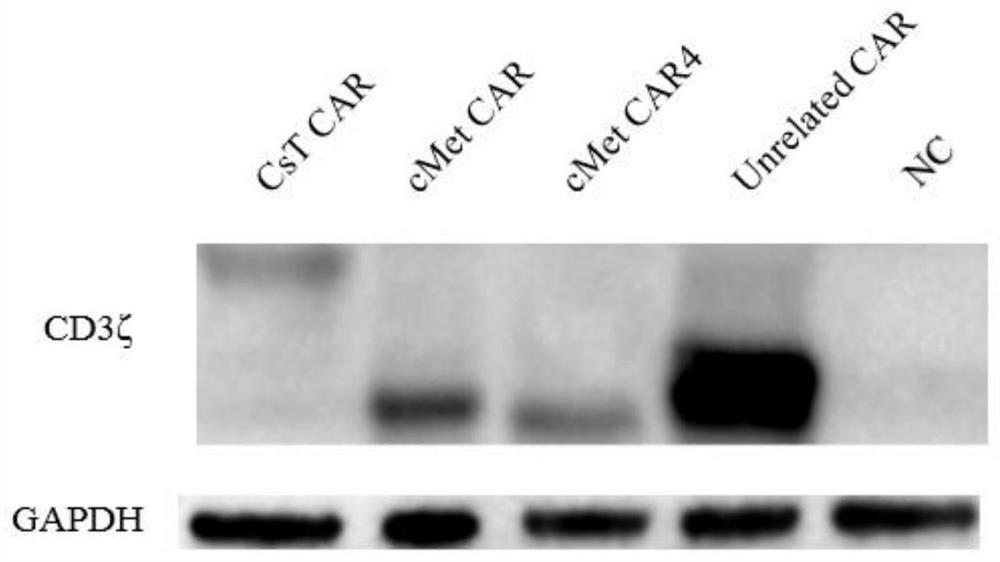
[0070] After transfection 1D and 2D, the fluorescent microscope was used to observe the GFP fluorescence expression after transfection, and X-293T cells were collected after transfection for 2D, and the total cell protein was extracted. The expression of exogenous CD3ζ was detected by Western Blot. like image 3 As shown, each CAR plasmid expressed exogenous CD3ζ....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com