Novel glutamine antagonists and uses thereof
An amino acid, selected technology, applied in the fields of peptides, organic chemistry, chemical instruments and methods, etc., can solve problems such as failure and hinder clinical development, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
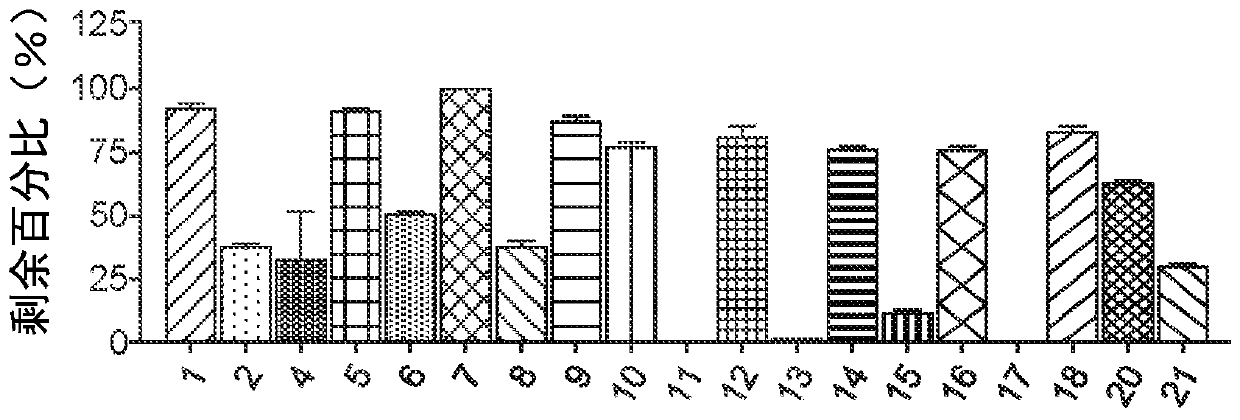
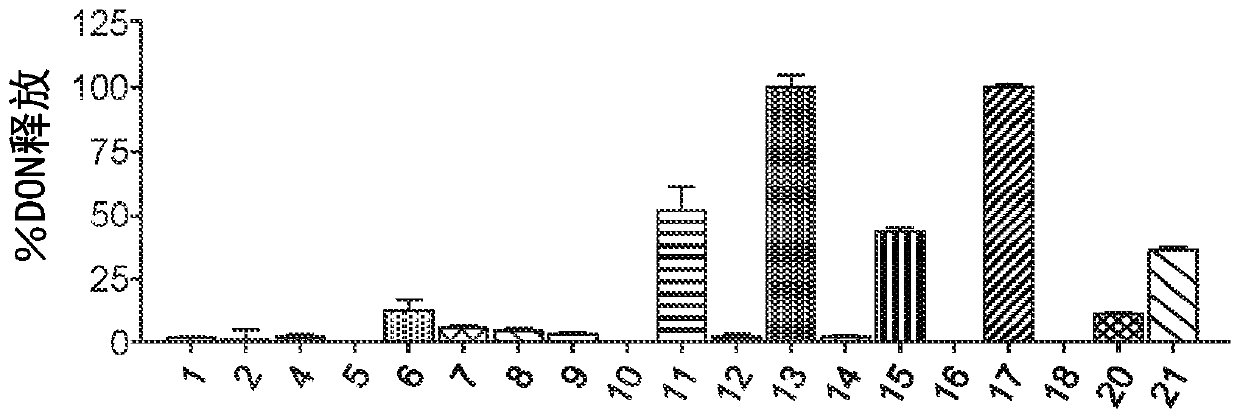
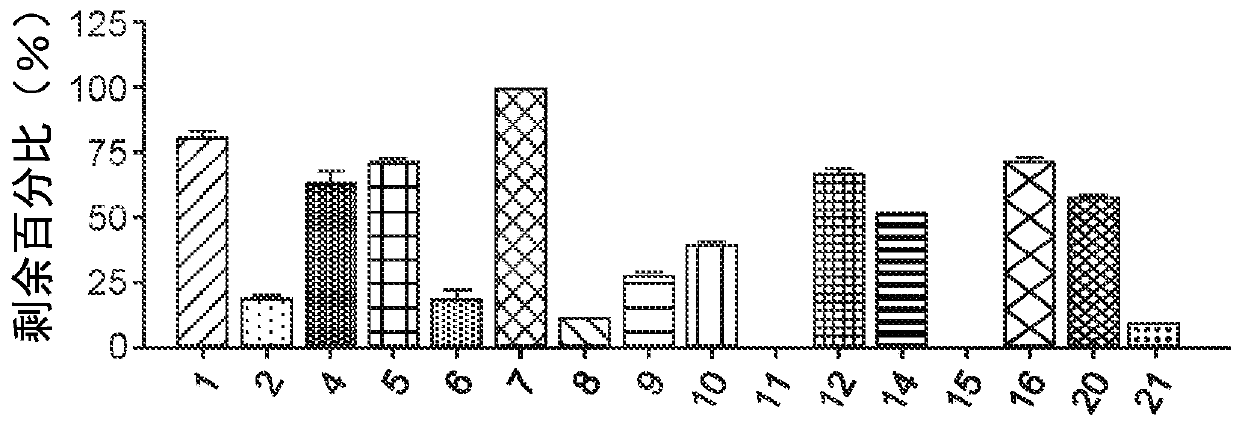
Image
Examples
Embodiment 1
[0274] Example 1 Isopropyl 2-(6-acetylamino-2-((tert-butoxycarbonyl)amino)-hexanoylamino)-6-diazo-5-oxohexanoate (compound 1).
[0275]
[0276] Boc-L-Lys(Ac)-OH (446 mg, 1.55 mmol, 1.1 equiv) and HBTU (641 mg, 1.69 mmol, 1.2 equiv) were suspended in anhydrous DCM (8 mL), and the suspension was cooled to 0 °C. DIEA (546 mg, 735 μL, 4.22 mmol, 3 equiv) was added. The reaction mixture was stirred for 5 min, then a solution of DONiPr (300 mg, 1.41 mmol, 1 eq) in anhydrous DCM (2 mL) was added via syringe over 5 min. Under an inert atmosphere, the reaction mixture was stirred at 0 °C for 0.5 h and at room temperature for 1 h. Then add DCM (30mL), wash with H 2 O (2×50mL), brine (50mL) washed the solution, and washed with MgSO 4 dry. The organic solvent was evaporated in vacuo. The residue was chromatographed on silica gel (DCM / MeOH, 25:1) to give the desired product 1 (535 mg, 79%) as a pale yellow amorphous solid.
[0277] 1 H NMR (400MHz, CDCl 3 ):1.22(3H,d,J=6.3),1.2...
Embodiment 2
[0283] Example 2 2-(2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-6-acetamide hexanoylamino)-6-diazo-5-oxohexanoic acid iso Propyl ester (Compound 2).
[0284]
[0285] Fmoc-L-Lys(Ac)-OH (6.35 g, 15.5 mmol, 1.1 equiv) and HBTU (6.41 g, 16.9 mmol, 1.2 equiv) were suspended in anhydrous DCM (80 mL), and the suspension was cooled to 0 ℃. DIEA (5.46 g, 7.35 mL, 42.2 mmol, 3 equiv) was added. The reaction mixture was stirred for 5 minutes, then a solution of DONiPr (3.00 g, 14.1 mmol, 1 equiv) in anhydrous DCM (20 mL) was added via syringe over 10 minutes. Under an inert atmosphere, the reaction mixture was stirred at 0 °C for 0.5 h and at room temperature for 1 h. Add DCM (30mL), wash with H 2 O (2×50mL), brine (50mL) washed the solution, and washed with MgSO 4 dry. The organic solvent was evaporated in vacuo. The residue was chromatographed on silica gel (CHCl 3 / MeOH, 30:1), the desired product 2 (5.72 g, 67%) was obtained as a pale yellow amorphous solid.
[0286] ...
Embodiment 3
[0292] Example 3 Isopropyl 2-(6-acetylamino-2-aminocaproylamino)-6-diazo-5-oxohexanoate (Compound 3).
[0293]
[0294] Compound 2 (5.00 g, 8.26 mmol, 1 equiv) was dissolved in anhydrous DCM (150 ml), and diethylamine (3.51 g, 4.97 mL, 41.3 mmol, 5 equiv) was added. The reaction mixture was stirred overnight (20 hours) at room temperature. The solvent was evaporated and the crude product was purified on silica gel to afford 2.91 g (92%) of compound 3 as a yellow solid.
[0295] 1 H NMR (400MHz, CDCl 3):1.20(3H,d,J=6.0),1.21(3H,d,J=6.0),1.33–1.53(4H,m),1.56–1.68(1H,m),1.72–1.84(1H,m) ,1.92(3H,s),1.91–2.02(1H,m),2.09–2.21(1H,m),2.28–2.48(2H,m),3.10–3.25(2H,m),3.43–3.73(3H, m), 4.43 (1H, td, J = 8.1, 4.7), 4.97 (1H, septet, J = 6.0), 5.46 (1H, bs), 6.51 (1H, bs), 8.05 (1H, d, J = 7.7).
[0296] 13 C NMR (101MHz, CDCl 3 ):21.76,22.57,23.22,27.29,29.06,29.71,33.92,36.70,39.06,51.84,54.53,55.13,69.40,170.66,171.24,174.15,194.15.
[0297] IR (KBr): 3432, 2110, 1731, 1635,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com