Niraparib intermediate, preparation method and application thereof, and synthesis method of niraparib
A synthesis method and intermediate technology, which is applied in the preparation of cyanide reaction, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of inability to racemize, recycle and reuse, and improve the overall utilization rate of materials and process safety , The effect of reducing the waste of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
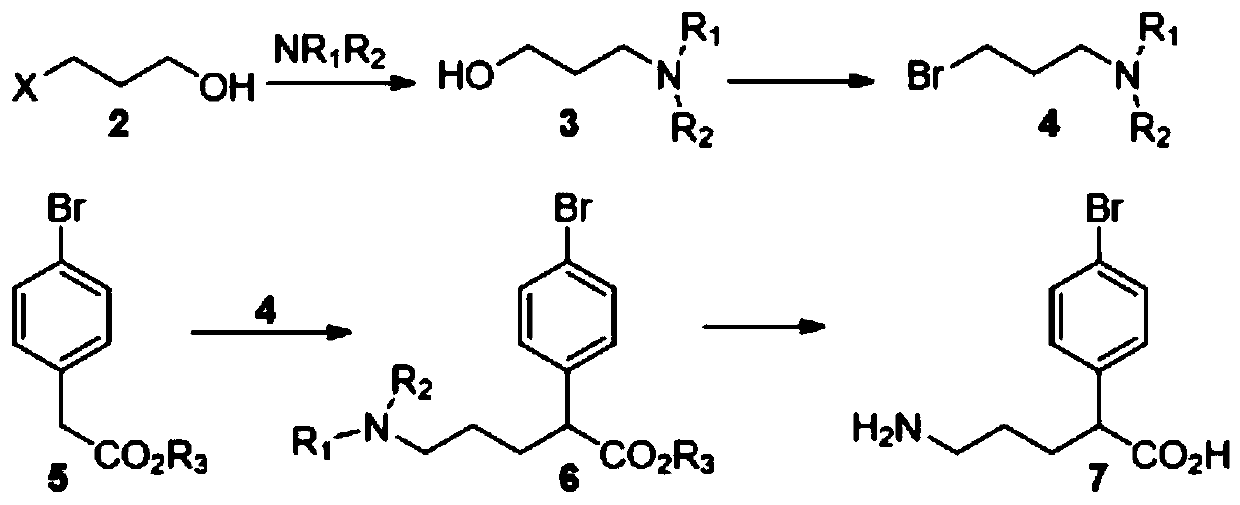
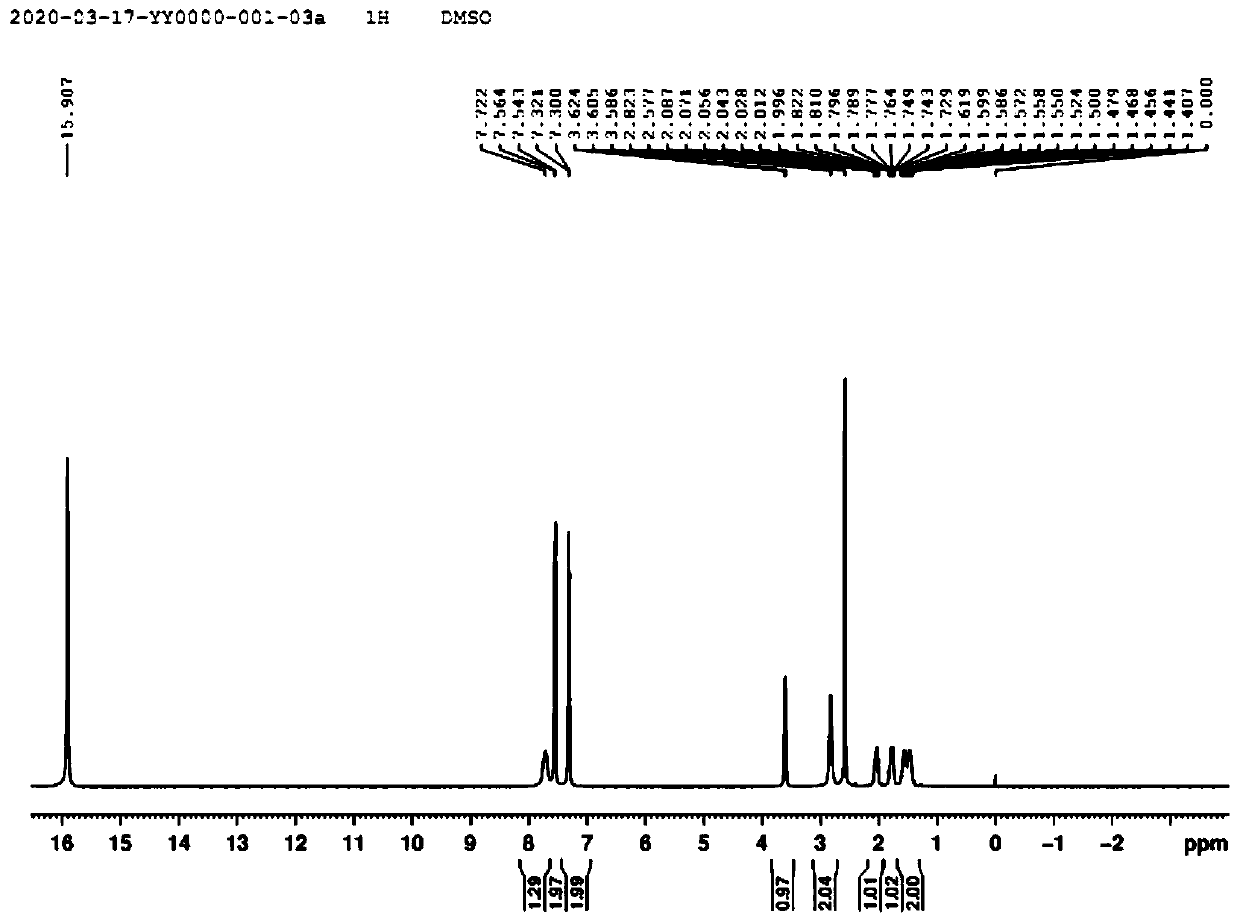
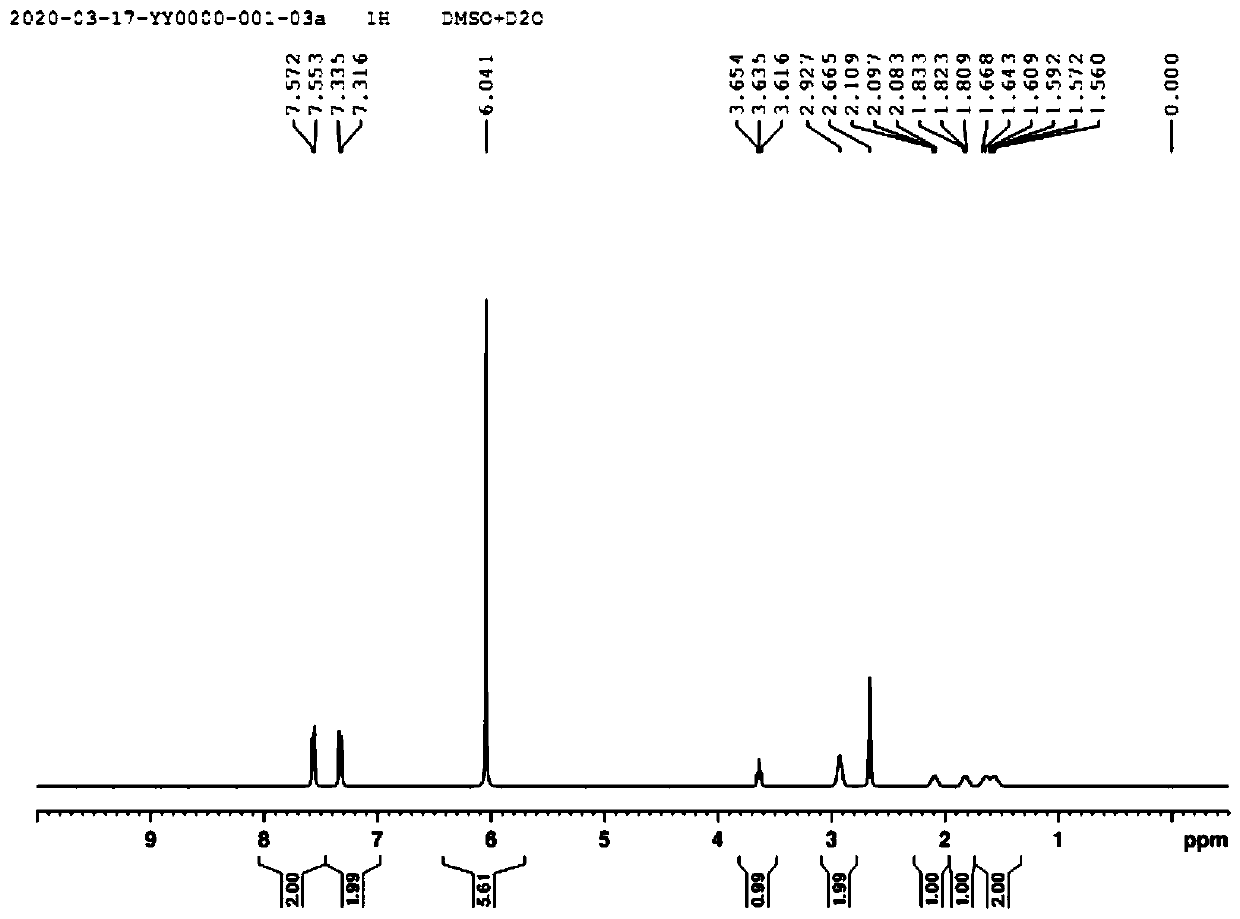
[0065] The synthetic method of niraparib key body N-Boc-(3S)-(4-bromophenyl)piperidine, first prepare compound 7 (alpha-(3-aminopropyl)-p-bromophenylacetic acid), synthetic route Such as figure 1 shown, including the following steps:
[0066] (1) Preparation of intermediate compound 3 (N-(3-hydroxypropyl) phthalimide)
[0067] Mix 125.4ml 3-chloropropanol (compound 2), 287.5g phthalamide potassium salt, and 369ml DMF, then keep warm at 120-125°C for reaction, stir for 4-5 hours, check that the reaction is complete, evaporate under reduced pressure to remove most of the Add part of DMF, add 540ml of water, 700ml of EA, stir and separate the layers, back extract the aqueous phase with 150ml of EA once, combine the organic phase, wash with saturated brine 200ml*4, dehydrate the organic phase, evaporate to dryness, add 1200ml of MTBE and heat to reflux to dissolve the crude product After cooling down to room temperature naturally, the ice water was cooled to 0-5 degrees and filt...
Embodiment 2
[0091] The preparation method of new compound 7 (α-(3-aminopropyl)-p-bromophenylacetic acid), and different from Example 1, in step (2), the intermediate compound 4 (N-(3-bromopropyl base) phthalimide) is prepared as follows: 245.3g of compound 3 is mixed in 1141.5ml of DCM, covered with a drying tube, cooled to 0-5 degrees, and slowly added dropwise with 125ml of phosphorus tribromide (FW: 270.7, 2.85 , 1.1eq), remove the ice water after the dropwise addition, stir at room temperature for 1h, TLC detects that the conversion is complete, add 1000ml of semi-saturated brine dropwise, stir for ten minutes to separate the layers, wash the organic phase with 900ml of saturated sodium bicarbonate and the pH of the aqueous phase is not low In 7, dry the organic phase, evaporate to dryness under reduced pressure to obtain the crude product, add 781ml of anhydrous methanol, heat to 58 degrees to dissolve, cool to 0-5 degrees with ice water, stir for half an hour and filter to obtain 211...
Embodiment 3
[0094] The synthesis method of N-Boc-(3S)-(4-bromophenyl)piperidine (compound 1), wherein the nitrogen in compound 7 is realized by introducing dibenzylamine.
[0095] (1) Preparation of intermediate compound (N-(3-hydroxypropyl) dibenzylamine)
[0096] We carried out the synthesis of (N-(3-hydroxypropyl)dibenzylamine) with reference to the method for the first step in Example 1, and the product yield was 81.2%.
[0097] (2) Preparation of intermediate compound (N-(3-bromopropyl) dibenzylamine)
[0098] We carry out bromination with reference to the second step method in Example 1, and the product yield is 82.3%
[0099] (3) Preparation of intermediate compound 7 (methyl p-bromophenylacetate)
[0100] Drop into DMSO 1250ml in the reaction bottle, slowly add potassium tert-butoxide 75.9g, in two dropping funnels, respectively dress embodiment 1 step (3) gained compound 5 144.4g, the DMSO (145ml) solution and embodiment 3 steps (2 ) of the obtained compound N-(3-bromopropyl)dib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com