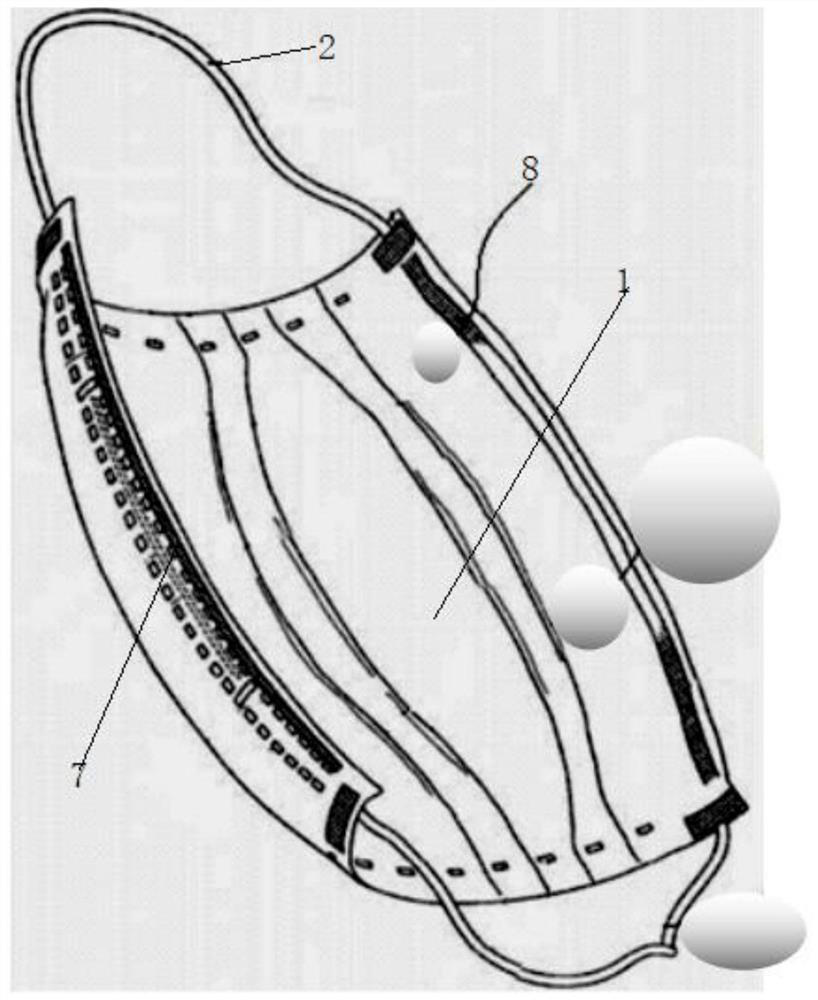
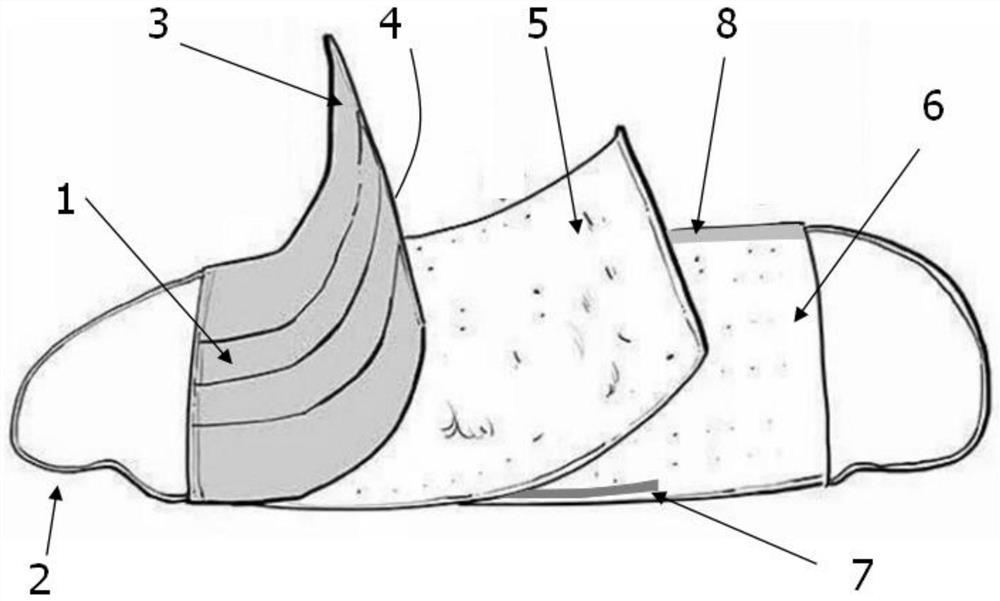
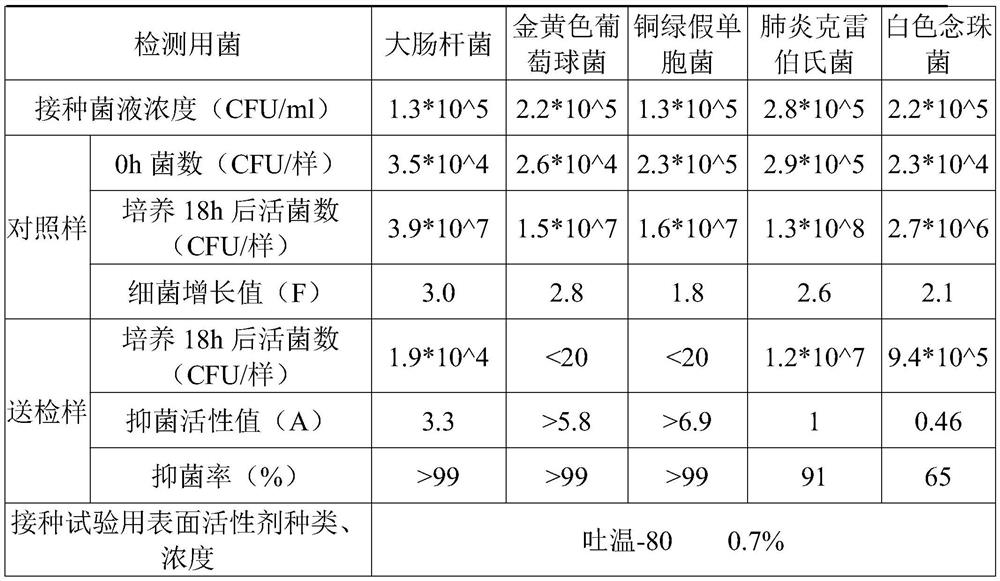
Disposable medical antibacterial mask
A disposable mask technology, applied in application, protective clothing, fiber treatment, etc., can solve the problems that have not kept pace with the times, and basically continue to be used many years ago, with low sterilization and anti-virus capabilities, to increase and antibacterial capabilities, strengthen Antibacterial ability, hygienic and safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Step 1: 20 parts by weight of water-based fluorocarbon resin DF-01, 1.5 parts of dodecyl alcohol ester Texanol film-forming aid, 0.3 part of TEGO-810 defoamer, 0.4 part of TEGO-245 substrate wetting agent , 0.4 parts of BYK-333 leveling agent, 0.5 parts of sodium carboxymethylcellulose thickener with a molecular weight of 150,000, 13 parts of CR828Tio2 coordination modified fungicide, 0.4 parts of sodium tripolyphosphate pH regulator and 0.3 parts of sorbic acid Potassium anticorrosion and antifungal agent for material preparation.
[0052] Step 2. Under stirring at 1050 rpm, add 1.5 parts of dodecyl alcohol ester Texanol coalescing aid to 20 parts of water-based fluorocarbon resin DF-01 at a rate of 0.25 g / s, and then stir at 1150 rpm Under the speed of rotation, stir for 25 minutes;
[0053] Step 3: Stir at 980 rpm, add 0.3 parts of TEGO-810 defoamer, 0.4 parts of sodium tripolyphosphate pH regulator and 0.3 parts of potassium sorbate antiseptic Under the rotating s...
Embodiment 2
[0063] Step 1. According to parts by weight, 25 parts of ETERFLON-4302, 5 parts of Dow Chemical PGDA coalescent, 0.4 parts of TEGO-810 defoamer, 0.3 parts of TEGO-245 substrate wetting agent, 1 part of BYK-333 Leveling agent, 0.4 parts of carboxymethylcellulose sodium thickener with a molecular weight of 160000, 10 parts of CR828Tio2 coordination modified fungicides, 0.3 parts of sodium tripolyphosphate pH regulator and 0.4 parts of potassium sorbate antiseptic and antifungal agent Prepare materials.
[0064] Step 2. Under stirring at 1050 rpm, add 5 parts of Dow Chemical PGDA film-forming aid to 25 parts of ETERFLON-4302AF at a rate of 0.20 g / s, then stir at 1200 rpm for 20 minute;
[0065] Step 3: Stir at 1000 rpm, add 0.4 parts of TEGO-810 defoamer, 0.3 parts of sodium tripolyphosphate pH regulator and 0.4 parts of potassium sorbate antiseptic Under the rotating speed, stir for 15 minutes;
[0066] Step 4, under stirring at 1000 rpm, continue to add 10 parts of CR828Tio2...
Embodiment 3
[0076] Step 1. According to parts by weight, 18 parts of water-based fluorocarbon resin DF-01, 3 parts of dodecyl alcohol ester Texanol film-forming aid, 0.3 part of TEGO-810 defoamer, 0.4 part of BNK-NSF047 substrate wetting agent , 0.6 parts of BYK-333 leveling agent, 0.6 parts of sodium carboxymethylcellulose thickener with a molecular weight of 190000, 6 parts of CR828Tio2 coordination modified fungicide, 0.3 parts of sodium tripolyphosphate pH regulator and 0.3 parts of sorbic acid Potassium anticorrosion and antifungal agent for material preparation.
[0077] Step 2. Under stirring at 950 rpm, add 3 parts of dodecyl alcohol ester Texanol coalescence aid to 18 parts of water-based fluorocarbon resin DF-01 at a rate of 0.22 g / s, and then stir at 1150 rpm Under the speed of rotation, stir for 22 minutes;
[0078] Step 3: Stir at 1020 rpm, then add 0.3 parts of TEGO-810 defoamer, 0.3 parts of sodium tripolyphosphate pH regulator and 0.3 parts of potassium sorbate antiseptic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com