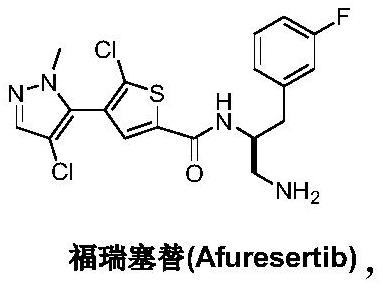
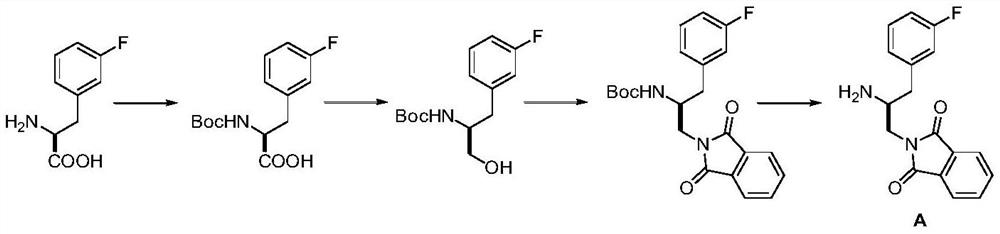
Preparation method of Afuresertib
A reaction and amino technology, which is applied in the design of organic synthesis routes and the preparation of raw materials and intermediates, can solve the problems of lengthening reaction steps and affecting the final yield, and achieve the effects of quality improvement, simple process and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 3-fluorophenylacetaldehyde (II) (6.9g, 50mmol), (2R)-2-methoxymethyl-1-amino-tetrahydropyrrole (6.5g, 50mmol) and 100mL of benzene into the reaction flask, Under stirring, raise the temperature to 80-85°C, pass through the water separator until no water drops appear, and then react for 6 hours. Lowered to room temperature, washed with saturated brine, dried, and concentrated under reduced pressure to give light yellow oil (2R)-2-methoxymethyl-N-[2-(3-fluorophenyl)ethylidene]-1- Amino-tetrahydropyrrole (III) 8.7g, yield 69.6%, EI-MS m / z: 251[M+H] + .
Embodiment 2
[0044] Add titanium tetrachloride (40mL, 40mmol, 1.0M dichloromethane solution), (2R)-2-methoxymethyl-N-[2 -(3-fluorophenyl)ethylidene]-1-amino-tetrahydropyrrole (III) (5.0g, 20mmol) and dichloromethane 50mL, after stirring for 30 minutes, trimethylsilylnitrile (3.0g , 30mmol) in 30mL of dichloromethane solution, kept at low temperature for 12 hours, and slowly rose to room temperature. Washed successively with saturated ammonium chloride solution and water, dried, and concentrated under reduced pressure to obtain light yellow oil ((2R)-2-methoxymethyl-N-[(1S)-2-(3-fluorophenyl) Methyl-2-acetonitrile]-1-amino-tetrahydropyrrole (IV) 4.1g, yield 74.0%, EI-MS m / z: 278[M+H] + .
Embodiment 3
[0046] Add (2R)-2-methoxymethyl-N-[(1S)-2-(3-fluorophenyl)methyl-2-acetonitrile]-1-amino-tetrahydropyrrole to the hydrogenation reaction flask (IV) (2.8 g, 10 mmol), palladium on carbon (0.14 g, 5% w / w) and methanol 50 mL. At 25-30°C, according to the hydrogenation reaction procedure, hydrogen gas was introduced under normal pressure, and the reaction was stirred for 4-6 hours. The catalyst was recovered by filtration, and the solution was concentrated under reduced pressure to obtain 1.5 g of a light colorless oil (αS)-α-amino-3-fluorophenylpropionitrile (V), with a yield of 91.5%, EI-MS m / z: 165[ M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com