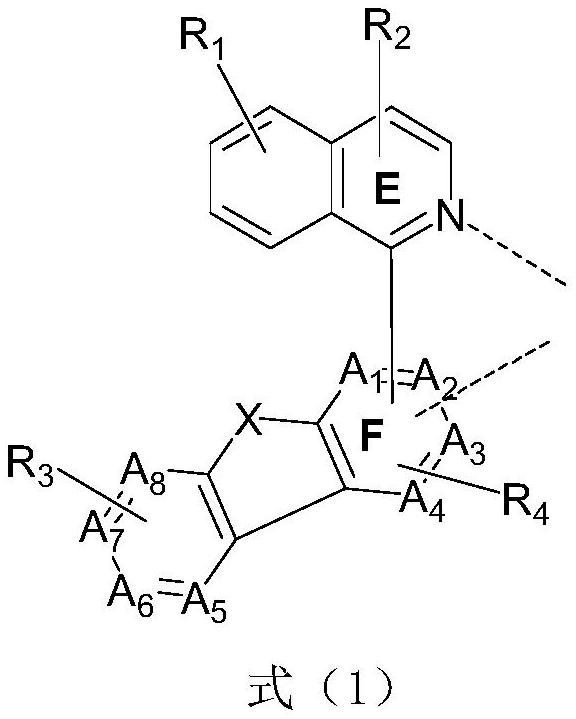
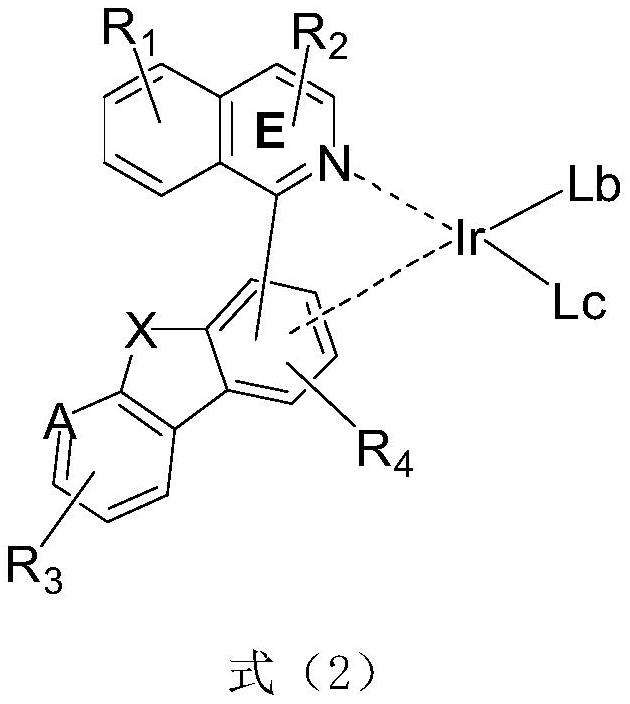
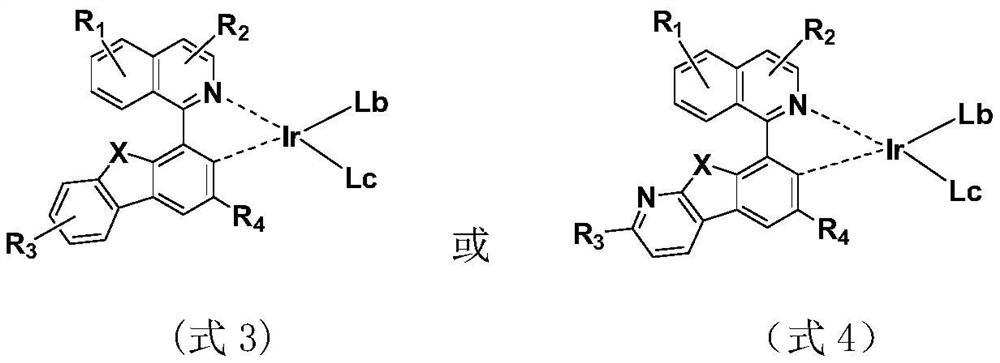
Metal complex and application thereof
A technology of metal complexes and compounds, which is applied in the direction of indium organic compounds, platinum group organic compounds, compounds containing group 8/9/10/18 elements of the periodic table, etc., can solve poor color saturation and device performance Unsatisfactory performance, high sublimation temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The synthesis of embodiment 1 compound A1
[0073] Synthesis of ligands L50 and La385:
[0074]
[0075] Synthesis of Compound L50:
[0076] Compound L1-1 (36.0g, 155.3mmol, 1.0eq), L1-2 (23.3g, 155.3mmol, 1.0eq), potassium carbonate (42.9g, 310.7mmol, 2.0eq) and dichlorodi-tert-butyl- (4-Dimethylaminophenyl) phosphopalladium (II) (1.02g, 1.5mmol, 0.01eq) was put into a 1L three-necked flask, and toluene (360ml), ethanol (96ml), deionized water (96ml) were added , vacuum, nitrogen replacement 3 times, under the protection of nitrogen, the temperature was raised to 80°C and the reaction was stirred for 6 hours. TLC monitoring (developing solvent: ethyl acetate / n-hexane=1 / 10), the raw material L1-1 was almost consumed. Cool to room temperature, separate the liquids, wash the organic phase with water (3*180ml), then extract the aqueous phase once with ethyl acetate (150ml), carry out silica gel suction filtration, wash with ethyl acetate until there is no obvious pro...
Embodiment 2
[0087] The synthesis of embodiment 2 compound A2
[0088] Synthesis of ligands La193 and La769:
[0089]
[0090] Synthesis of Compound L4:
[0091] Referring to the synthesis process and post-treatment purification method of compound La385, only the corresponding raw materials need to be changed. Mass spectrum: 366.2 (M+H).
[0092] Synthesis of Compound L5:
[0093] Referring to the synthesis process and post-treatment purification method of compound La385, only the corresponding raw materials need to be changed. Mass spectrum: 414.5 (M+H).
[0094] Synthesis of Compound A2
[0095]
[0096] Synthesis of compound A2-1:
[0097] Referring to the synthesis process and post-treatment purification method of compound A1-1, only the corresponding raw materials need to be changed. Mass spectrum: 712.3 (M+H).
[0098] Synthesis of Compound A2-2:
[0099] Referring to the synthesis process and post-treatment purification method of compound A1-2, only the corresponding ...
Embodiment 3
[0102] The synthesis of embodiment 3 compound A3
[0103]
[0104] Synthesis of compound A3:
[0105] Referring to the synthesis process and post-treatment purification method of compound A1, only the corresponding raw materials need to be changed to obtain the target compound A3 (3.11 g, 50.2%). Sublimated pure A3 (2.17 g, 69.7%) was obtained after sublimation and purification of 3.11 g of crude A3. Mass spectrum: 1193.5 (M+H). 1 H NMR (400MHz, CDCl 3 )δ8.38(d, J=20.0Hz, 2H), 7.94(dd, 2H), 7.72(m, J=5.0Hz, 2H), 7.55(d, J=15.0Hz, 4H), 7.46(m, 2H), 7.39(m, 4H), 7.31(m, 4H), 2.34(s, 6H), 1.88(m, 3H), 1.77(t, J=18.8Hz, 3H), 1.66(m, J=2.2 Hz,5H),1.31(m,4H),1.24(m,4H),1.01(m,J=5.7Hz,8H),0.94(m,12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com